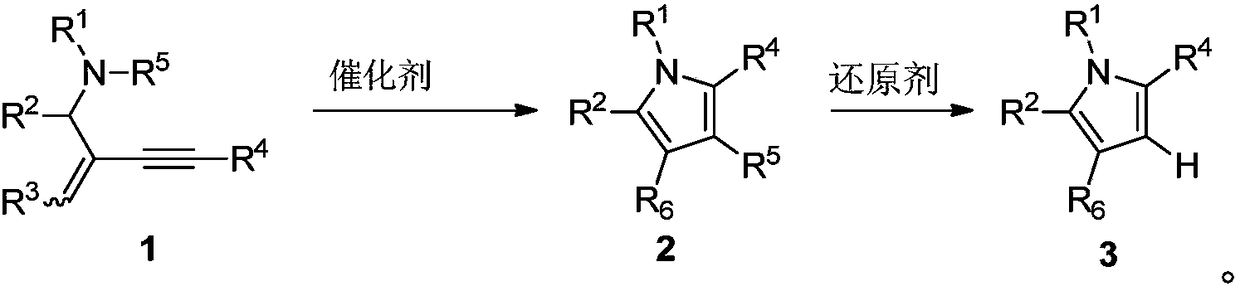
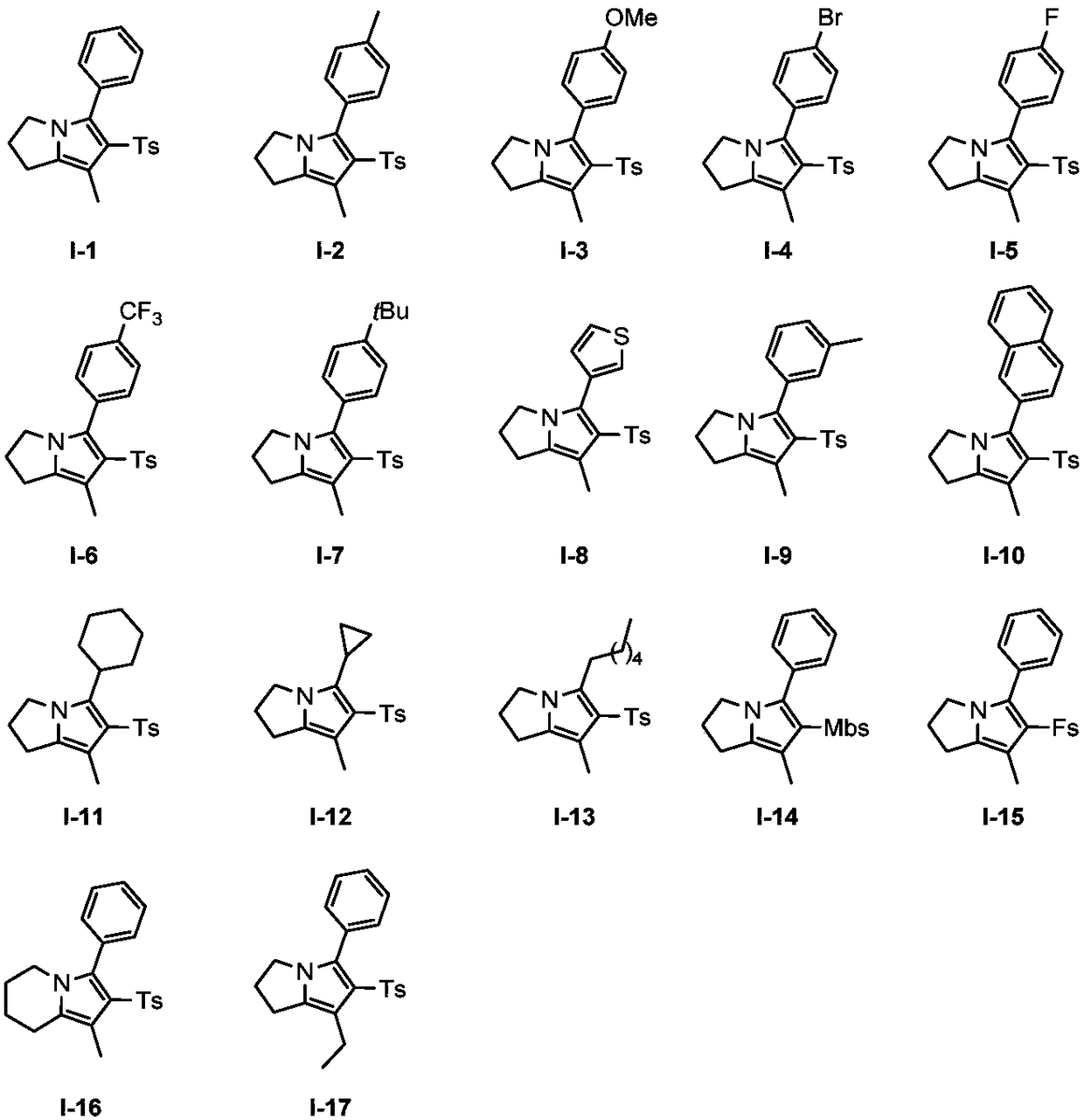
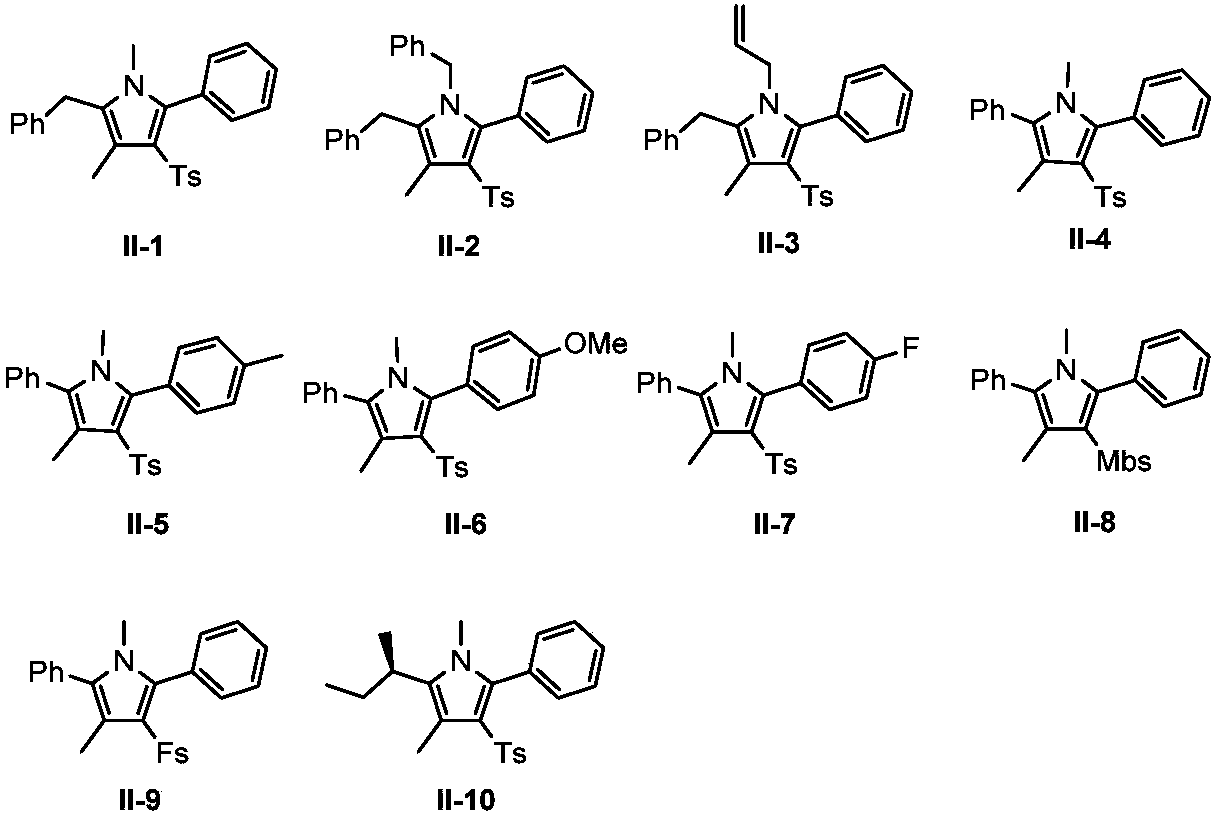
Preparation method of polysubstituted pyrrole compound
A compound and multi-substitution technology, which is applied in the field of preparation of multi-substituted pyrrole compounds, can solve the problems of severe conditions and poor chemical selectivity, and achieve the effects of high reactivity, wide application range and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of 2-{[(4-phenyl-1-en-3-yne)-2-butyl]}-1-p-toluenesulfonylpyrrole
[0054]
[0055] Add proline methyl ester hydrochloride (5g, 30.3mmol) in the round bottom flask of 250mL, p-toluenesulfonyl chloride (5.76g, 30.3mmol), then add 200mL methylene chloride as solvent in the flask, then Triethylamine (6.13 g, 60.6 mmol) was added dropwise thereto. After the dropwise addition, the reaction was stirred at room temperature for 20 h. After the reaction was complete, 200 mL of water was added to quench the reaction. Extract with dichloromethane, combine organic phase, after using 1M dilute hydrochloric acid washing, organic phase is dried with anhydrous magnesium sulfate, and rotary evaporation removes organic solvent, obtains the N-p-toluenesulfonylproline methyl ester of target (8.15g , yield 95%).
[0056] The N-p-toluenesulfonyl proline methyl ester (5g, 17.6mmol) obtained in the previous step was dissolved in anhydrous tetrahydrofuran (100mL), and N, O-dime...
Embodiment 2
[0060] Preparation of N,4-methyl-N-[(3-methylene-1,5-diphenyl-4-yne)-2-pentyl]benzenesulfonamide
[0061]
[0062] Add phenylalanine methyl ester hydrochloride (5g, 23.2mmol) in the round bottom flask of 250mL, p-toluenesulfonyl chloride (4.4g, 23.2mmol), then add 200mL methylene chloride as solvent in the flask, Then triethylamine (4.67 g, 46.4 mmol) was added dropwise thereto. After the dropwise addition, the reaction was stirred at room temperature for 20 h. After the reaction was complete, 200 mL of water was added to quench the reaction. Extract with dichloromethane, combine organic phase, after using 1M dilute hydrochloric acid washing, organic phase is dried with anhydrous magnesium sulfate, and remove organic solvent with rotary evaporation, obtain the N-p-toluenesulfonyl phenylalanine methyl ester of target (7.4 g, yield 96%).
[0063] In the round bottom flask of 50mL, add N-p-toluenesulfonyl phenylalanine methyl ester (3.1g, 9.3mmol), anhydrous potassium carbon...
Embodiment 3
[0068] Preparation of 7-methyl-5-phenyl-6-p-toluenesulfonyl-2,3-dihydro-1H-pyrine
[0069]
[0070] Under nitrogen protection, a magnetic stirrer was added to a clean round bottom flask, 1mL of 1,2-dichloroethane was added, and IPrAuCl (0.005mmol, 3.1mg), AgNTf 2 (0.005mmol, 1.9mg), stirred for 10min, then added 2-{[(4-phenyl-1-ene-3-yne)-2-butyl]}-1-p-toluenesulfonylpyrrole (0.1mmol, 35mg), reacted at 40°C for 8h. After the reaction, the reaction solution was filtered through short silica gel, the filtrate was concentrated, and separated by column chromatography to obtain the target product. The developer ratio is petroleum ether:ethyl acetate=5:1, and the final product is a white solid with a yield of 74%.
[0071] Its physical constant of the product that present embodiment makes is: 1 HNMR (CDCl 3,400MHz) 1 H NMR (400MHz, CDCl 3 )δ7.53(d, J=8.1Hz, 2H), 7.46–7.29(m, 5H), 7.15(d, J=8.0Hz, 2H), 3.71(t, J=7.1Hz, 2H), 2.79( t,J=7.2Hz,2H),2.42(q,J=7.2Hz,2H),2.36(s,3H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com