Oral insulin nanometer granular preparation and method for preparing same
A nanoparticle and insulin technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low bioavailability of insulin and easy to be trapped, and achieve good results. Hypoglycemic effect, blood sugar control, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Synthesis of chitosan quaternary ammonium salt (HTCC)
[0061] 2g chitosan was dissolved in 100mL of 2wt% acetic acid solution, heated to 80 o After C, slowly drop 5mL glycidyltrimethylammonium chloride (GTMAC) aqueous solution into the solution, 80 o The reaction was carried out at C for 24 h, and the resulting solution was cooled and precipitated twice in 10 times the volume of acetone. The precipitate was redissolved in double distilled water, dialyzed against water for 3 days, and freeze-dried to obtain the final product HTCC. Among them, the mass concentration of GTMAC aqueous solution is 20%, and the degree of quaternization of HTCC is 43%. In addition, by adjusting the volume of GTMAC to 2mL or 4mL, HTCC with quaternization degrees of 12% and 23% could be obtained, respectively.
Embodiment 2
[0062] Example 2 Synthesis of thiolated hyaluronic acid (HA-SH)
[0063] 1. Dissolve hyaluronic acid (HA) in double distilled water at a concentration of 5mg / mL, add ion exchange resin (the mass ratio of resin to hyaluronic acid is 3:1), stir for 0.5h, filter off the resin, and use tetrabutylhydrogen The pH value of the solution was adjusted to 7.04 with ammonium oxide, and lyophilized to obtain the product HA-TBA.
[0064] 2. Dissolve HA-TBA (1.5g) in DMSO at a concentration of 0.5mg / mL, and add 4-dimethylaminopyridine (0.35g), dithiodipropionic acid (2.4g), di-tert-dicarbonate Butyl ester (0.2mL), react at 45°C for 20h. After cooling to room temperature, dialyze against double distilled water to remove DMSO, then precipitate twice in 10 times volume of acetone, dissolve in double distilled water, dialyze against water for 3 days, and freeze-dry. Dissolve the lyophilized product (0.5g) in double distilled water, adjust the pH to 8.5, add dithiothreitol (0.5g), react for 3h,...
Embodiment 3
[0065] Example 3 Preparation of nanoparticles using FNC technology
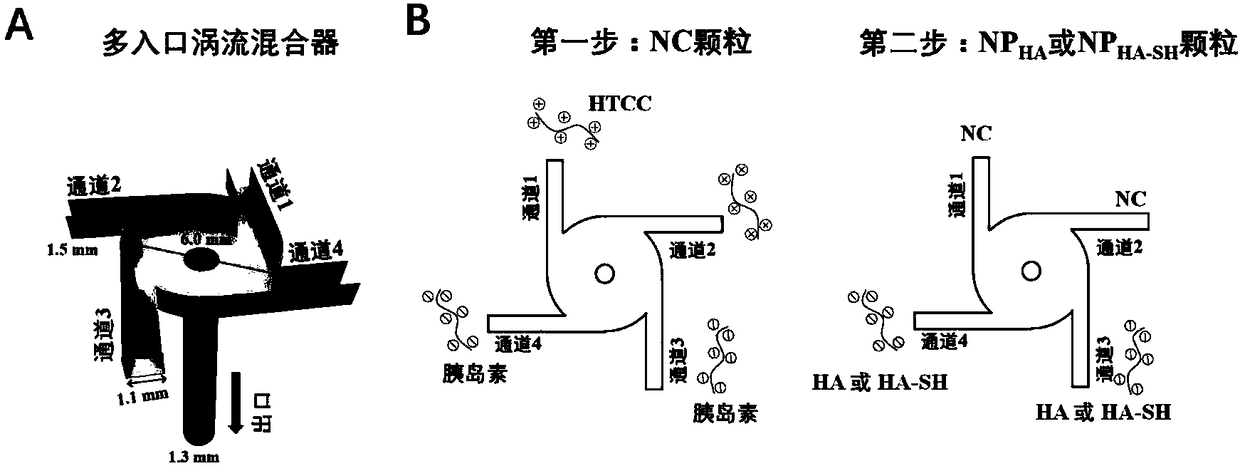
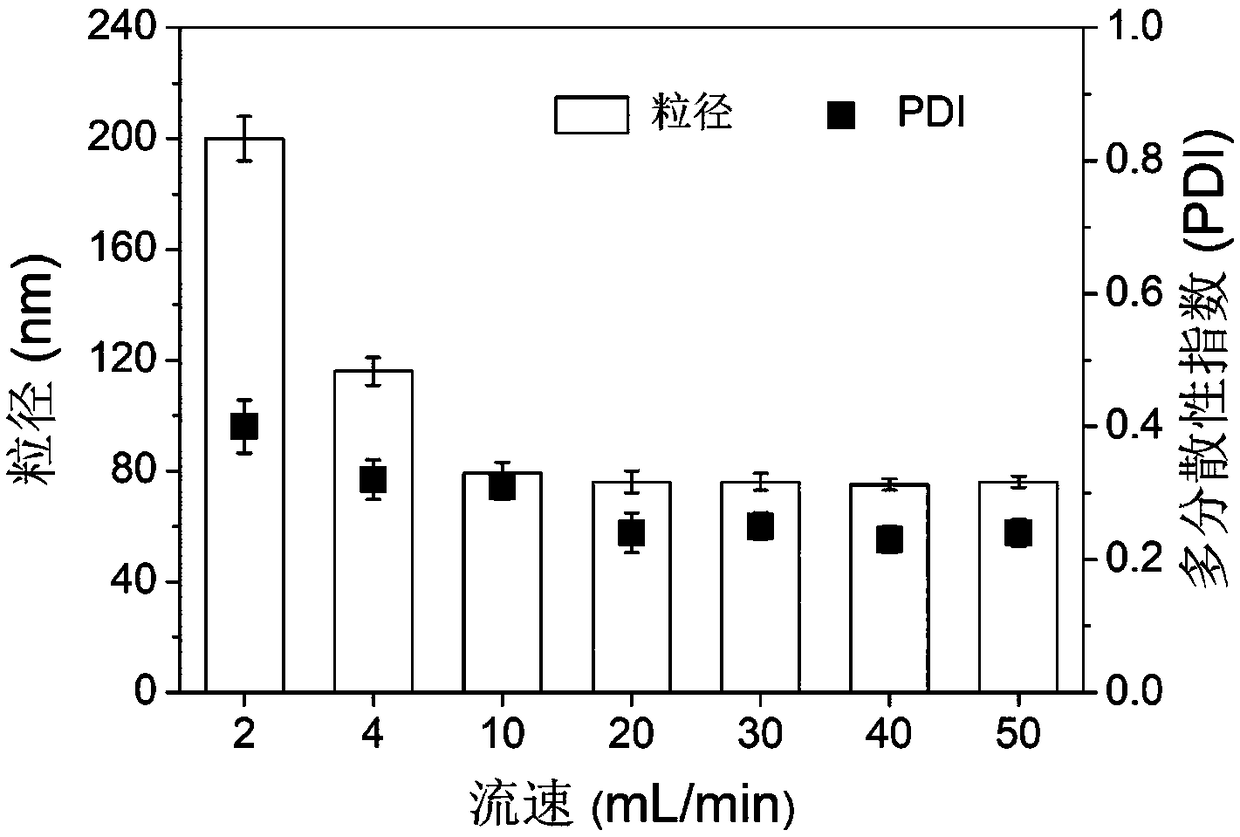
[0066] FNC technology is a technology for preparing drug-loaded nanoparticles through electrostatic interaction in water phase (recorded in the inventor's previous patent application number PCT / US2017 / 014080), and the preparation process is mainly carried out in a multi-entrance vortex mixer , with the characteristics of high throughput and strong controllability, the prepared nanoparticles are evenly distributed and small in particle size, and the difference between batches is small. Since there is no organic solvent involved in the whole process, it is especially suitable for the preparation of biological macromolecules such as proteins and nucleic acids. The multi-inlet vortex mixer ( figure 1 A, figure 1 B) comprising a first part at the top, a second part at the middle and a third part at the bottom, the first part, the second part and the third part are cylinders with the same diameter; the first part...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com