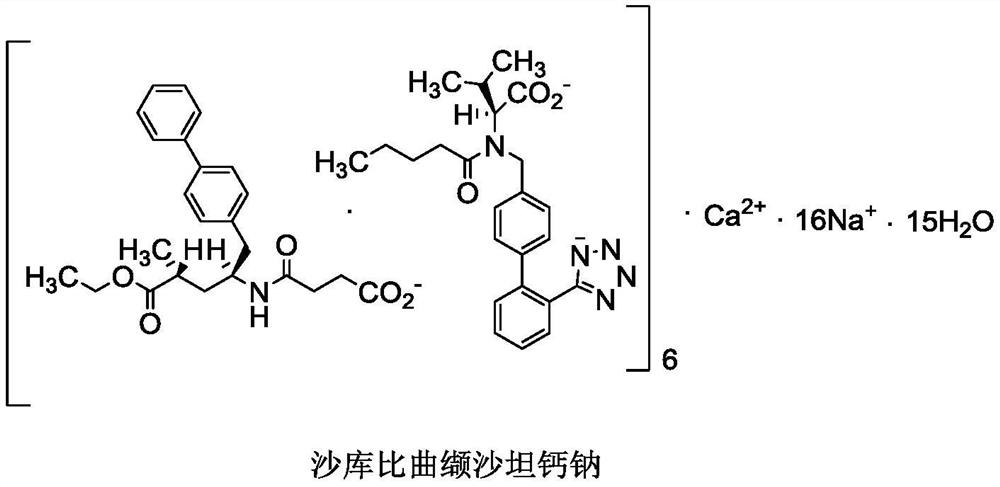
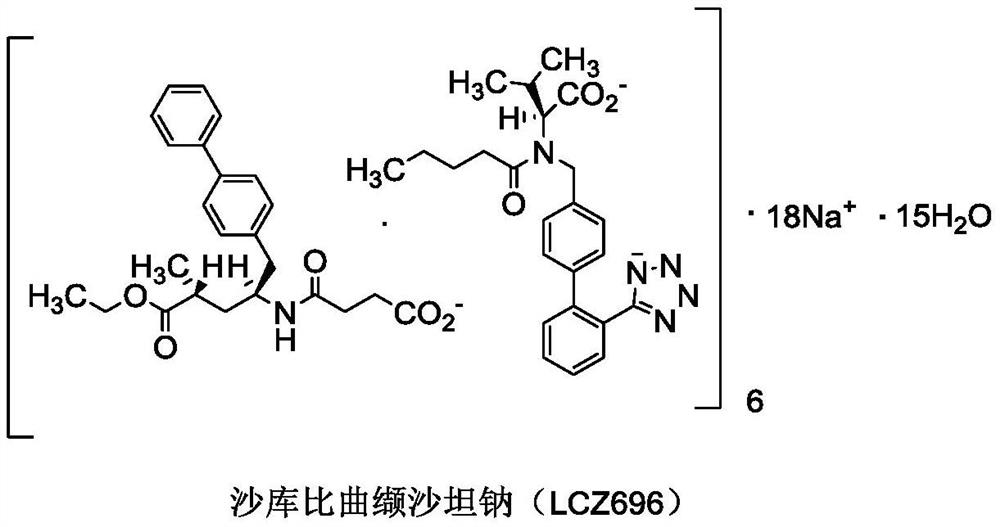
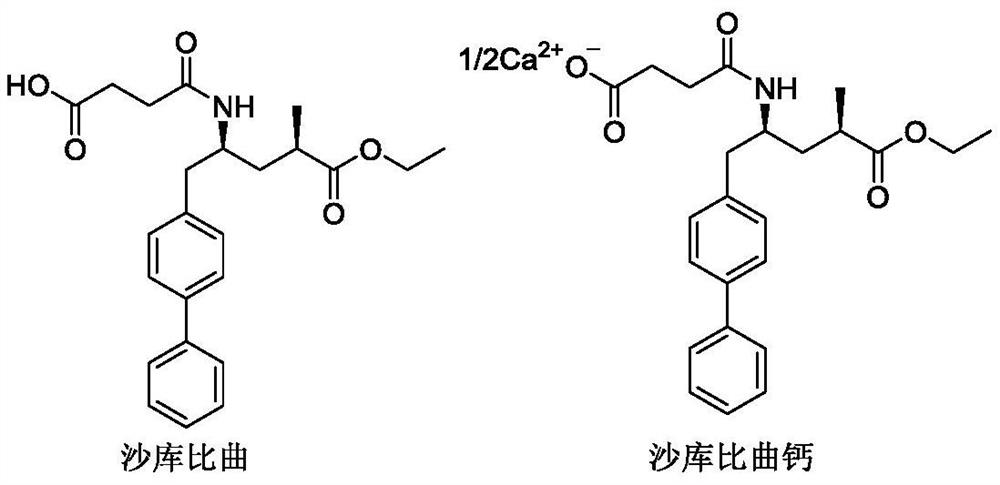
A preparation method of sacubitril valsartan complex and/or co-crystal key intermediate sacubitril calcium
A sacubitril and compound technology, which is applied to the preparation of pharmaceutical intermediates, the preparation field of sacubitril-valsartan complex and/or co-crystal key intermediate sacubitril calcium, can solve the problem of low efficiency, Problems such as long steps and unfavorable amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Compound II ((2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-pentanoic acid ) preparation
[0037] Add (R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-pentane to the hydrogenation vessel under stirring -2-enoic acid (compound III) (75g, 0.20mol), 10% palladium on carbon (Pd / C) (3.0g) and 750ml of ethanol, hydrogen was passed through and kept under pressure (1.0MPa, 25°C) for 20 hours. Filtrate, concentrate to dryness, add an appropriate proportion of isopropyl acetate / petroleum ether for recrystallization, and obtain a white solid (compound II) after drying, the specific data is shown in the table below. 1 H NMR (DMSO-d 6 )δ12.01(s,1H),7.63(d,2H,J=7.6Hz),7.56(d,2H,J=8.0Hz),7.47-7.43(m,2H),7.35(d,1H,J =7.2Hz),7.24(d,2H,J=8.0Hz),6.73(d,1H,J=8.8Hz),3.67-3.66(m,1H),2.68(d,2H,J=6.8Hz), 2.45-2.42(m,1H),1.78-1.71(m,1H),1.32(s,9H),1.40-1.32(m,1H),1.06(d,3H,J=6.8Hz).
Embodiment 2
[0040] Example 2 Preparation of Compound I ((2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-amino-2-methyl-pentanoic hydrochloride)
[0041] Add (2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-pentane to the reaction flask under stirring Acid (compound II) (45g, 0.12mol), ethanol 225ml, thionyl chloride (69.3g, 0.58mol) was added dropwise at 5°C, after the addition was completed, the temperature was raised to 40°C to react for 4 hours. Concentrate to dryness, beat with petroleum ether, filter, and dry to obtain 39.5 g of off-white solid (Compound I), with a mass yield of 87.8%. 1 H NMR (DMSO-d 6 )δ8.33(s,3H),7.68-7.64(m,4H),7.49-7.45(m,2H),7.38-7.36(m,3H),4.01-3.96(m,2H),3.38-3.36( m,1H),3.12-3.08(m,1H),2.85-2.74(m,2H),1.89-1.83(m,1H),1.66-1.60(m,1H),1.09(t,3H,J=12.4 Hz), 1.07 (d, 3H, J=5.2Hz). HPLC 99.8%, isomer≤0.2%.
[0042]
Embodiment 3
[0043] Example 3 Shakubitra calcium (4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5 Preparation of -oxopent-2-yl)amino)-4-oxobutyrate calcium)
[0044] Add (2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-amino-2-methyl-pentane hydrochloride (compound I) into the reaction flask under stirring (36g, 0.10mol), succinic anhydride (9.7g, 0.10mol) and DMF 90ml, add an appropriate amount of calcium-containing alkali (mixture of one or more kinds) in batches at 0-10°C, after adding, heat up to 25°C for reaction 4 hours. Add water, beat, filter, and get crude product. Add an appropriate proportion of organic solvent and water to the mixed system for recrystallization, filter, and dry to obtain an off-white solid (Sacubitronic Calcium), with all isomers ≤ 0.1%, and other data are shown in the table below.
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com