Di(4-benzoindole)sulfone derivative and preparation method and application thereof
A technology of indole derivatives and indole benzene, which is applied in chemical instruments and methods, instruments, analytical materials, etc., can solve the problems of life overlap and other problems, and achieve the effects of shortened attenuation life, simple synthesis, and convenient detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of compound 2
[0040]Add 5-nitroindole (4.86 g, 30 mmol), 4,4'-difluorodiphenylsulfone (3.56 g, 14 mmol) and potassium carbonate (19.35 g, 140 mmol) into a 100 mL round bottom flask , under the protection of nitrogen, inject 70 mL of N-methylpyrrolidone, react at 140 °C for 4 hours, cool down to room temperature, pour the reaction solution into 500 mL of ice water, filter with suction, wash the filter cake repeatedly with water and petroleum ether, to obtain Gray-green solid 2, 97% yield. 1H NMR (400 MHz, DMSO) δ (ppm): 8.69 (d, J = 2.3Hz, 1H), 8.33 – 8.23 (m, 2H), 8.10 (dd, J = 9.2, 2.3 Hz, 1H), 8.03 ( d, J =3.4 Hz, 1H), 8.01 – 7.93 (m, 2H), 7.83 (d, J = 9.2 Hz, 1H), 7.07 (d, J = 3.3Hz, 1H).
[0041] (2) Synthesis of compound 1
[0042] Add bis[4-(5-nitroindole)phenyl]sulfone (10 mmol) and 300 mL ethanol to a 500 mL round bottom flask, followed by stannous chloride dihydrate (100 mmol), under nitrogen protection Reflux for 20 hours, cool to room te...
Embodiment 2
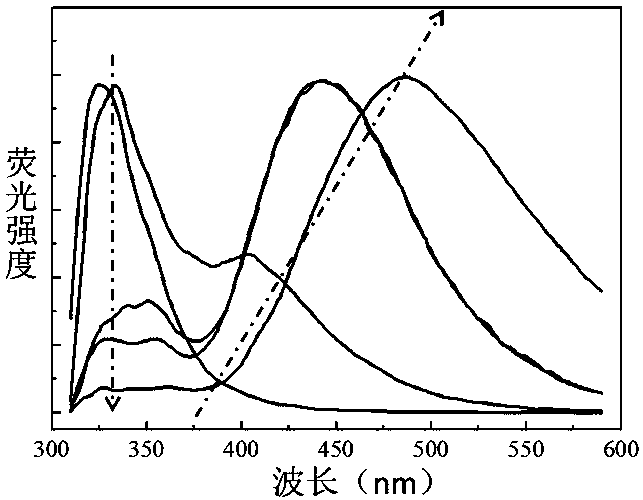
[0048] Compound I obtained above was dissolved in n-hexane, toluene, dichloromethane, tetrahydrofuran, and N,N-dimethylformamide respectively to prepare a 0.05 μmol / L solution. Add 2mL of the solution to a 1 cm×1 cm×4 cm stoppered cuvette, and test its fluorescence emission spectrum, λ ex = 300 nm, the result is as figure 2 shown. As the polarity of the solvent increased, the shift of the fluorescence emission peak remained unchanged, and its intensity gradually weakened, while the emission peak of the delayed fluorescence gradually red-shifted, and its intensity gradually increased.
Embodiment 3
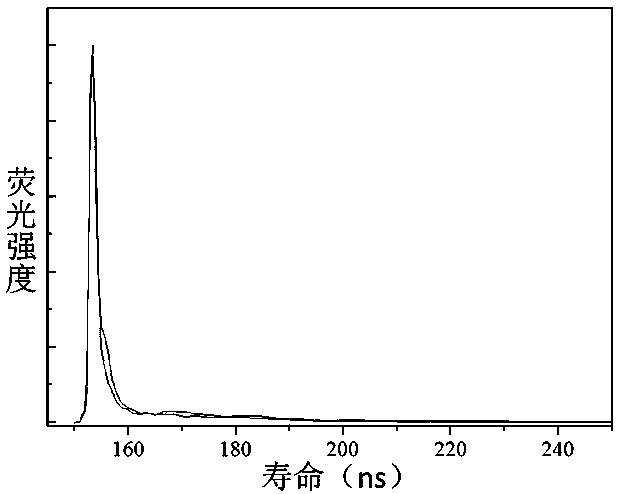
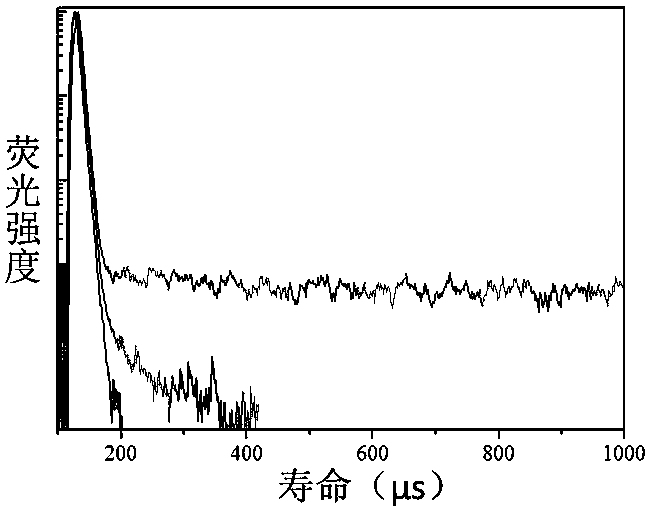
[0050] Compound I obtained above was dissolved in n-hexane and toluene, respectively, to prepare a 0.05 μmol / L solution. Add 2mL of the solution to a stoppered cuvette of 1 cm×1 cm×4 cm, and test its fluorescence decay lifetime, λ ex = 300 nm, the result is as image 3 shown. The fluorescence lifetime remains constant with increasing solvent polarity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com