Taurine substituted bodipy fluorescent compound and preparation method and application thereof
A fluorescent compound, the technology of fluoborofluorescein, which is applied in the field of taurine-substituted fluoborofluorescein fluorescent compounds and its preparation, can solve the problems of low atom economy, unfriendly organometallic reagents, and lengthy steps, etc., and achieve high fluorescence Effects of quantum yield, increased diversity, ease of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] TB-a (2,3,6,-triiodo-8-(4-methylphenyl)-5-taurine substituted flubororine) and TB-b (8-(4-methylphenyl )-3,5-bis-taurine substituted borofluores) Synthesis
[0055]
[0056] (1) Add pyrrole (67.1g, 1mol) and 4-methylbenzaldehyde (6.0g, 50mmol) into a dry light-proof reactor at room temperature under nitrogen protection, and after stirring for 10 minutes in the dark, add 1.0mL three Fluoroacetic acid, after the dropwise addition was completed, the reaction was stopped after stirring for 4 hours. The reaction was quenched with 20 mL of 0.1 M aqueous NaOH and extracted with 100 mL of ethyl acetate. The organic phase was washed twice with water, dried with anhydrous Na2SO4, filtered, the solvent was removed, and 3,4-dimethylpyrrole was recovered under reduced pressure. The residue was washed twice with water, dried with anhydrous Na2SO4, filtered, the solvent was removed, and the pyrrole derivative was recovered under reduced pressure. The residue was subjected to col...
Embodiment 2
[0059] TC-a (3-iodo-8-(4-methoxyphenyl)-5-taurine substituted borofluorene) and TC-b (2,6,-diiodo-8-(4-methyl Synthesis of oxyphenyl)-3,5-bistaurine substituted flubororine)
[0060]
[0061] (1) Pyrrole (67.1g, 1mol) and 4-methoxybenzaldehyde (7.0g, 50mmol) were added to a dry reactor at room temperature under nitrogen protection, and after stirring for 10min in the dark, 1.0mL was added dropwise to the system After the addition of trifluoroacetic acid was completed, the reaction was stopped after stirring for 4 hours. The reaction was quenched with 20 mL of 0.1M aqueous NaOH and extracted with 100 mL of ethyl acetate. After washing the organic phase twice with water, the 2 SO 4 Dry, filter, remove the solvent, and recover 3,4-dimethylpyrrole under reduced pressure. The residue was washed twice with water, and washed with anhydrous Na 2 SO 4 Dry, filter, remove the solvent, and recover the pyrrole derivative under reduced pressure. CH 2 Cl 2 / Petroleum ether / Et 3...
Embodiment 3
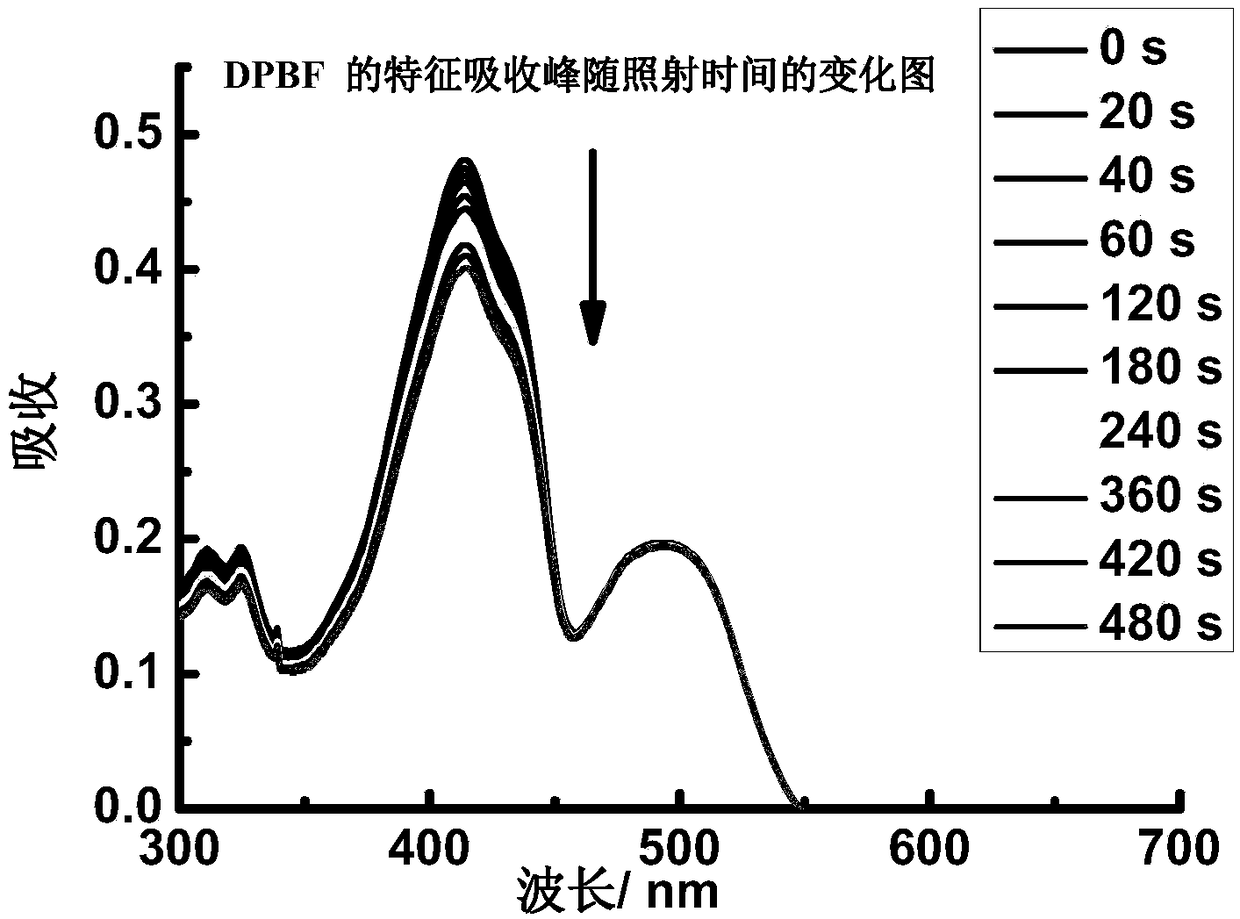
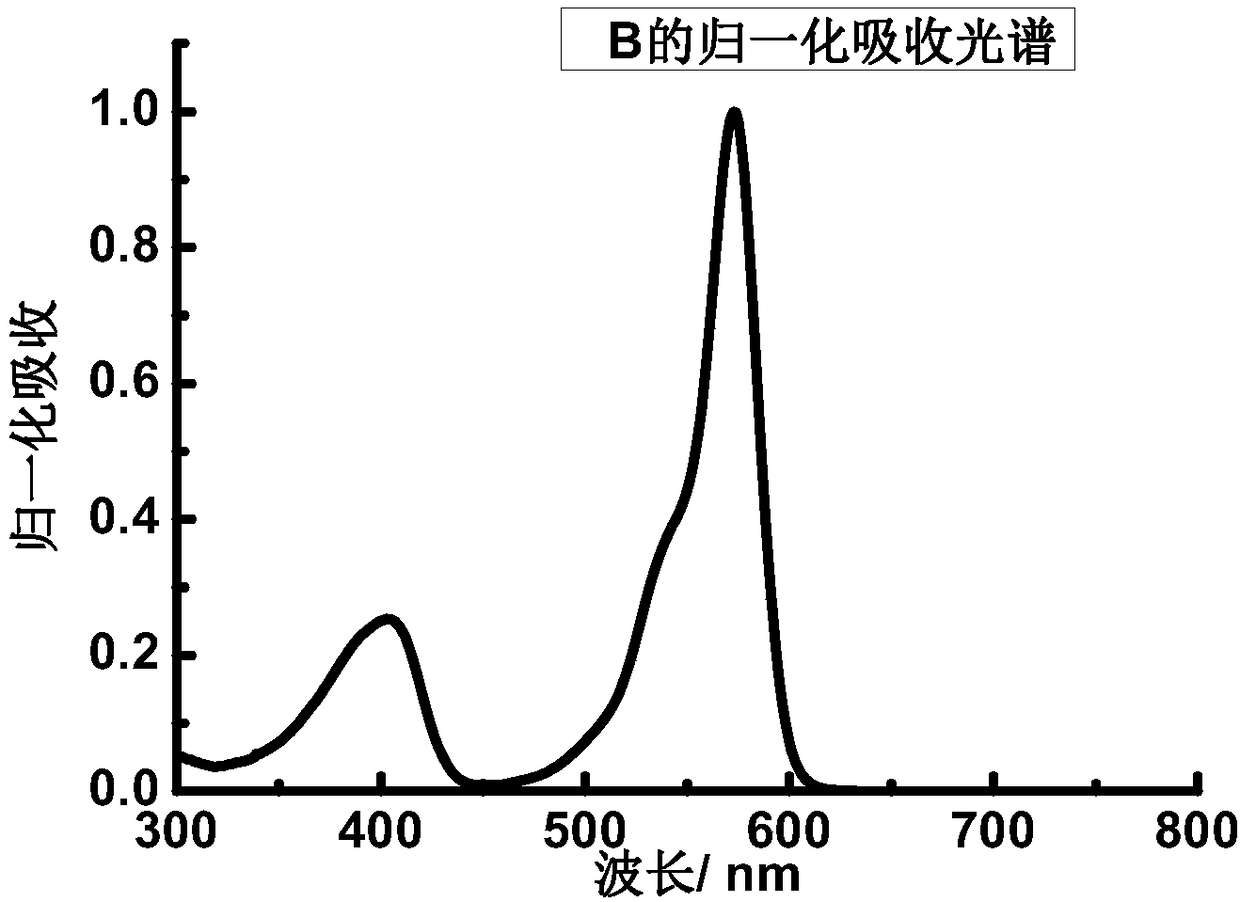
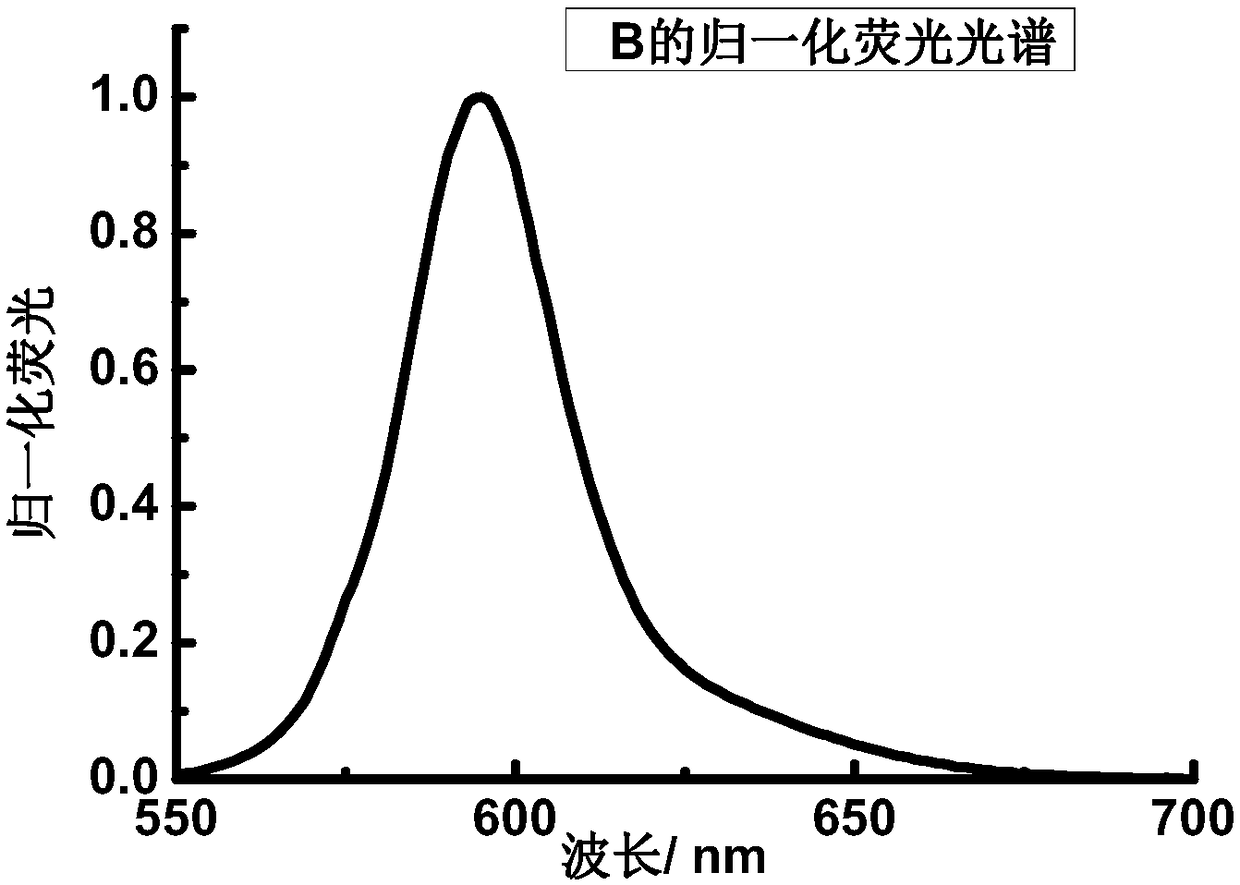
[0064] Dissolve B in THF to make 5.0×10 -6 mol / L solution, take 2.5mL and put it into a cuvette, and measure the ultraviolet-visible-near-infrared absorption and fluorescence emission spectra. The maximum absorption peak of the absorption spectrum of B is located at 573nm, and the molar extinction coefficient reaches 2.7×10 5 m -1 cm -1 ( figure 1 ). The maximum absorption peak of the fluorescence emission spectrum is located at 595nm ( figure 2 ), the quantum yield is 0.11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com