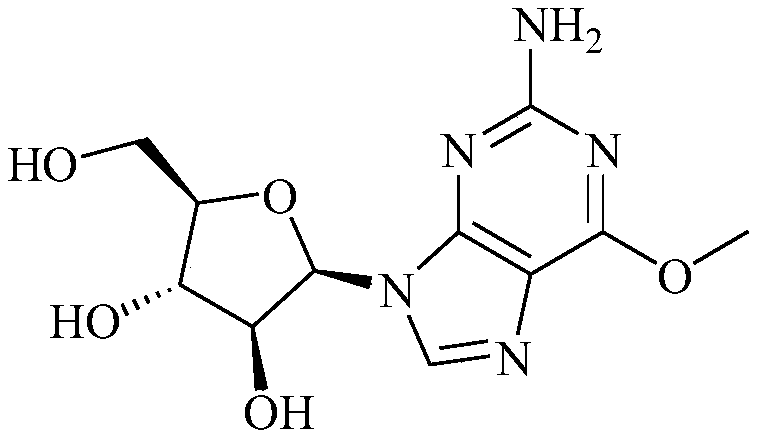
A kind of preparation method of nelarabine
A technology of nelarabine and amino, which is applied in the field of chemical preparation of nelarabine, can solve the problems of high raw material price, low total yield, long route, etc., and achieve the effect of easy control of reaction, short reaction route and safe reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
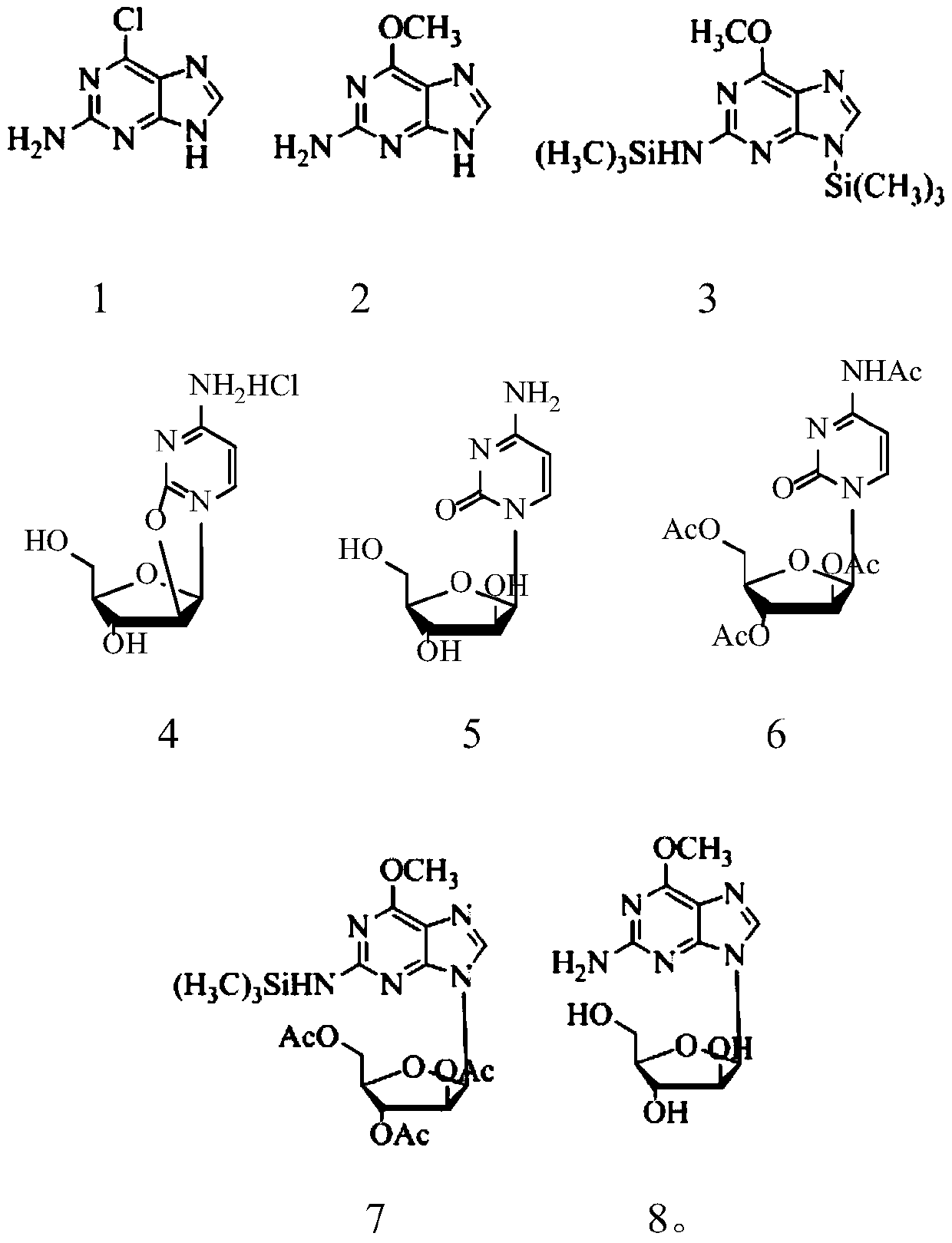
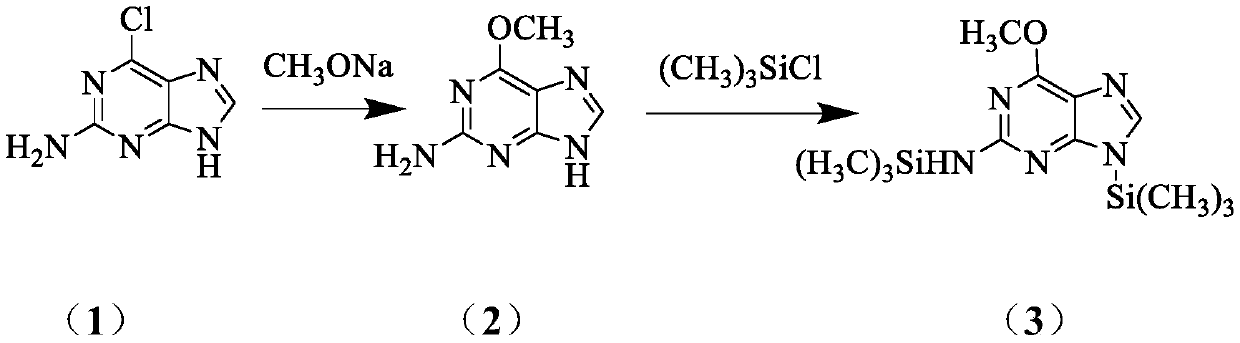
[0045] Preparation of 2-amino-6-methoxypurine (2)
[0046] Add 51.0g of 2-amino 6-chloropurine (1) and 34.0g of sodium methoxide into 500ml of methanol, stir to dissolve, heat in an oil bath at 67°C, reflux for 10h, and monitor the progress of the reaction by TLC. After the reaction was completed, the solvent was evaporated to produce a large amount of white solid, which was dissolved by adding 500ml of water, then adjusted to pH 7.5 with dilute hydrochloric acid solution, and a large amount of white solid was precipitated, left to stand for 30min and then filtered to obtain 44.4g of filter cake with a yield of 98.0%.
Embodiment 2
[0048]Preparation of silylated product (3) of 2-amino6-methoxypurine
[0049] Add 45.3g of 2amino-6-methoxypurine (2) into 500ml of 1,2-dichloroethane, stir for 10min, but it does not dissolve. Then 66.0 g of trimethylchlorosilane and 61.0 g of triethylamine were added, and the reaction was stirred at 60° C. for 6 to 7 hours, and the reaction progress was monitored by TLC. After the reaction, the insoluble matter was filtered out, and the filtrate was spin-dried to obtain 72.4 g of a reddish-brown oily substance 6-methoxy-N,9-bis(trimethylsilyl)-9H-purin-2-amine, with a yield of 78.0% .
[0050] Change the ratio of 6-methoxyguanine (2):trimethylchlorosilane:triethylamine to 1:5:5, and other conditions are the same as in Example 2. 69.5 g of reddish-brown syrupy substance 6-methoxy-N,9-bis(trimethylsilyl)-9H-purin-2-amine was obtained, with a yield of 71.1%.
[0051] The organic solvent was changed to dichloromethane, the reaction temperature was changed to 38° C., the react...
Embodiment 3
[0053] Preparation of cytarabine (4)
[0054] Add 52.9g of cyclocitidine hydrochloride into 500ml of water, stir to dissolve, then adjust the pH value of the solution to 12.0-14.0 with sodium hydroxide solution, stir and react at 25°C for 10h, and monitor the reaction progress by TLC. After the reaction, the water was evaporated to obtain a large amount of white solid, which was then dissolved in 500ml of methanol, filtered, and the filtrate was evaporated to dryness to obtain 43.8g of white powdery solid cytarabine, m.p.: 212-214°C. Yield 90.1%.
[0055] Change the reaction temperature to 60°C and the reaction time to 4h. Other conditions are the same as embodiment three. 44.8 g of white powdery solid cytarabine was obtained, m.p.: 213-214°C. Yield 92.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com