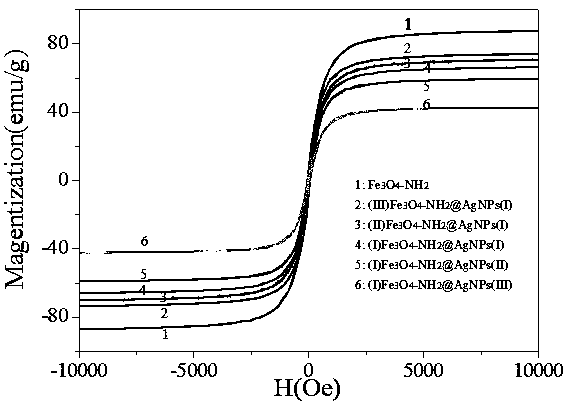
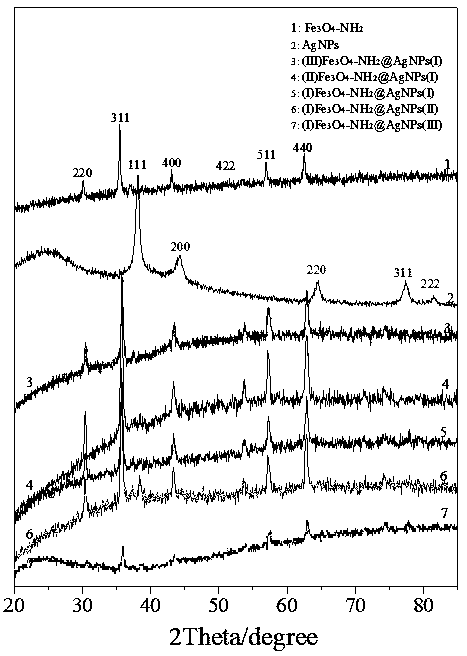
Preparation method and application of Fe3O4-NH2@AgNPs composite material
A fe3o4-nh2, composite material technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, water/sludge/sewage treatment, etc., can solve mutagenic, teratogenic, pollution, etc. problem, to achieve the effect of stable performance, low cost and energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Soak 10g of waste tea in 100mL of distilled water, boil at 60°C for 5min, filter out the extract, and set aside. waste tea extract and 1 mmol·L -1 The silver nitrate solution was mixed at a volume ratio of 1:4, stirred in a water bath at 60°C until the solution changed from yellow to dark brown, and the reaction was terminated. Place at room temperature for 24 hours, centrifuge at a high speed (8000r / min) for 15 minutes, put the precipitate into a glass bottle after centrifugation, dry at 80°C, and crush to obtain a brown solid, namely AgNPs, for later use;
[0027] (2) 2.0 g anhydrous sodium acetate, 1.0 g FeCl 3 ·6H 2 O and 7.0 g of 1,6-hexanediamine were dissolved in 30 mL of ethylene glycol, stirred vigorously until the mixed solution was clear, then transferred to a polytetrafluoroethylene autoclave, reacted at 160–200 °C for 6 h, and cooled to room temperature. After the product was repeatedly washed with water and ethanol, it was separated from the superna...
Embodiment 2
[0030] The five marked composite materials were used as catalysts, and hydrogen peroxide was used to catalyze the oxidation degradation of ethyl violet, malachite green and basic fuchsin aqueous solutions under natural light (this example takes ethyl violet as an example). After optimizing the experimental conditions (pH, time, temperature, usage of composite materials and hydrogen peroxide), measure the absorbance of the solution and calculate its degradation rate (each group of experiments was repeated three times to take the average value). The degradation rate of ethyl violet reached 99.1% within 30 minutes, and the degradation rate still reached about 98% after 10 times of recycling. The degradation rate of malachite green reaches 97%, and the degradation rate of basic fuchsin reaches 98%.
[0031] Catalytic degradation of ethyl violet, Fe 3 o 4 -NH 2 The appearance of the @AgNPs composite basically does not change much ( Figure 4 ), indicating that the prepared comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com