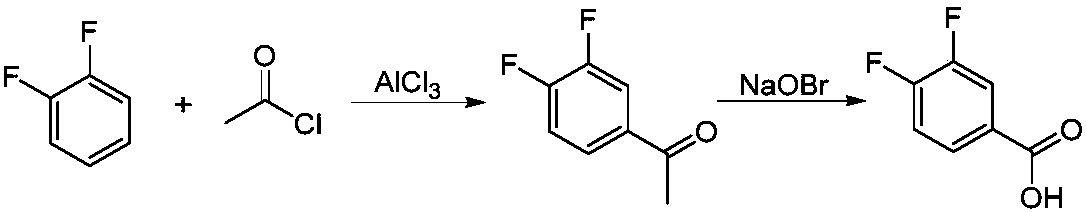
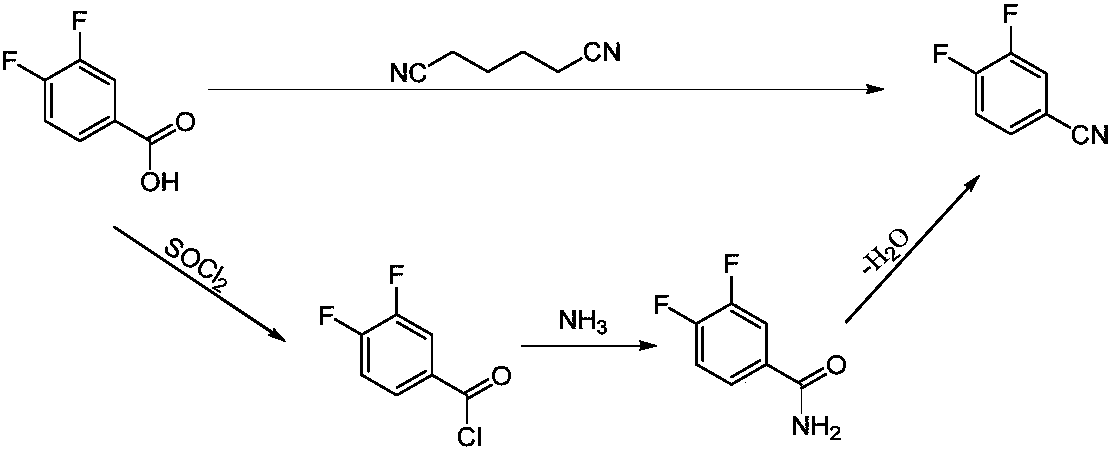
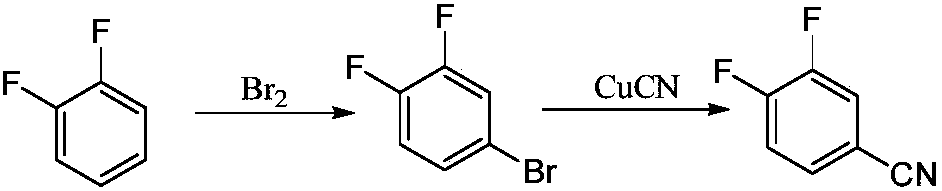
3,4-difluorobenzonitrile preparation method
A technology of difluorobenzonitrile and dichlorobenzonitrile, which is applied in the field of preparation of 3,4-difluorobenzonitrile, can solve the problems of high reaction temperature, high energy consumption in the production process, and high price of o-difluorobenzene, and can reduce the The effect of reaction temperature, reducing the generation of three wastes and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Preparation of catalyst bis-(N,N'-1,3-dimethyl-2-imidazolinyl)-ammonium chloride salt
[0041] Put 114g (1mol) of 1,3-dimethyl-2-imidazolidinone and 300g of toluene into the dry reaction flask, slowly add 100g (0.34mol) of triphosgene (0.34mol) and 200g of toluene to the reaction flask under the condition of 0-5°C Solution, react at room temperature for 2 hours after the dropwise addition, then slowly add ammonia (20g, 1.18mol)-toluene 100g solution dropwise to the reaction system at 0-5°C, after the dropwise addition, raise the temperature to 80°C to react 6 After the reaction, the toluene was removed to obtain the intermediate 1,3-dimethyl-2-imidazolinimine, which was set aside.
[0042]Put 114g (1mol) of 1,3-dimethyl-2-imidazolidinone and 300g of toluene into another dry reaction flask, and slowly add 100g (0.34mol) of toluene 200g to the reaction flask under the condition of 0-5°C Solution, react at room temperature for 2 hours after the dropwise addition, then ...
Embodiment 2
[0047] Embodiment 2 utilizes recovered catalyst and solvent to apply (eighth time) to prepare 3,4-difluorobenzonitrile
[0048] Drop into the DMI-catalyst solution (and add DMI to 300g) and anhydrous Potassium Fluoride (170g, 2.93mol) that embodiment 1 reclaims in the reaction flask of drying band rectification column, then add toluene 150g reflux and divide water about 1 Hours, after the water separation is completed, the toluene is evaporated and recovered for the next application, and then 3,4-dichlorobenzonitrile (200g, 1.16mol), bis-(N,N'-1,3-dimethyl- 2-imidazolinyl)-ammonium chloride salt (8g, 0.032mol), PEG2000 (3g) and sodium metabisulfite (3g), the temperature was raised to 200-215°C for 4-5 hours, and the rectification column received 3,4-di The crude product of fluorobenzonitrile was 150 g, and the crude product was subjected to secondary rectification to obtain 140 g of 3,4-difluorobenzonitrile product with a purity of 99% and a molar yield of 87%.
[0049] Cool ...
Embodiment 3
[0051] Put 250 g of 1,3-dimethyl-2-imidazolinone (DMI) and anhydrous potassium fluoride (170 g, 2.93 mol) into a dry reaction flask with a rectification column, then add 100 g of toluene to reflux and divide the water for about 2 Hours, after the water separation is finished, the toluene is evaporated and recovered for the next application, and then 3,4-dichlorobenzonitrile (200g, 1.16mol), the bis-(N,N'-1,3- Dimethyl-2-imidazolinyl)-ammonium chloride salt (40g, 0.16mol), polyacrylamide (10g) and sodium thiosulfite (20g), the temperature was raised to 190-200°C for 5 hours, and the rectification column received The crude product of 3,4-difluorobenzonitrile was 160g, and the crude product was subjected to secondary rectification to obtain 145g of 3,4-difluorobenzonitrile product with a purity of 99% and a molar yield of 90%.
[0052] Cool and filter after the reaction, rinse the filter cake with DMI, collect the filtrate containing the catalyst and directly apply it to the next...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com