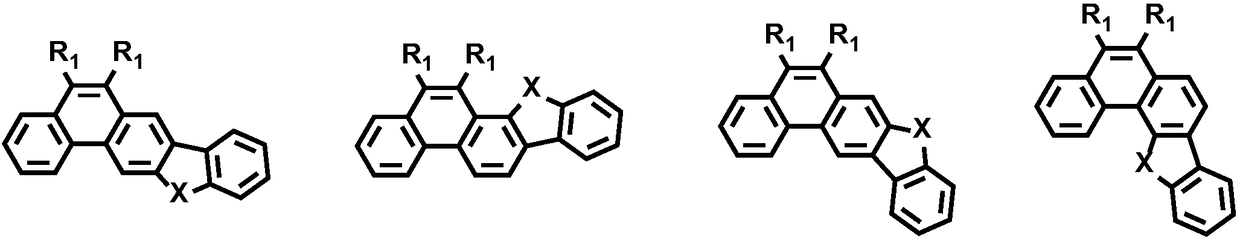
Five-member parallel-ring phenanthrene unit containing monomers and polymers and preparation methods and application thereof
A polymer and phenanthrene-containing technology, applied in the direction of hydrocarbon production from oxygen-containing organic compounds, chemical instruments and methods, preparation of halogenated hydrocarbons, etc., can solve the problems that limit the electroluminescence performance of polymer light-emitting materials, and achieve strong electron transport Effects of performance, good solubility, high fluorescence quantum yield and carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
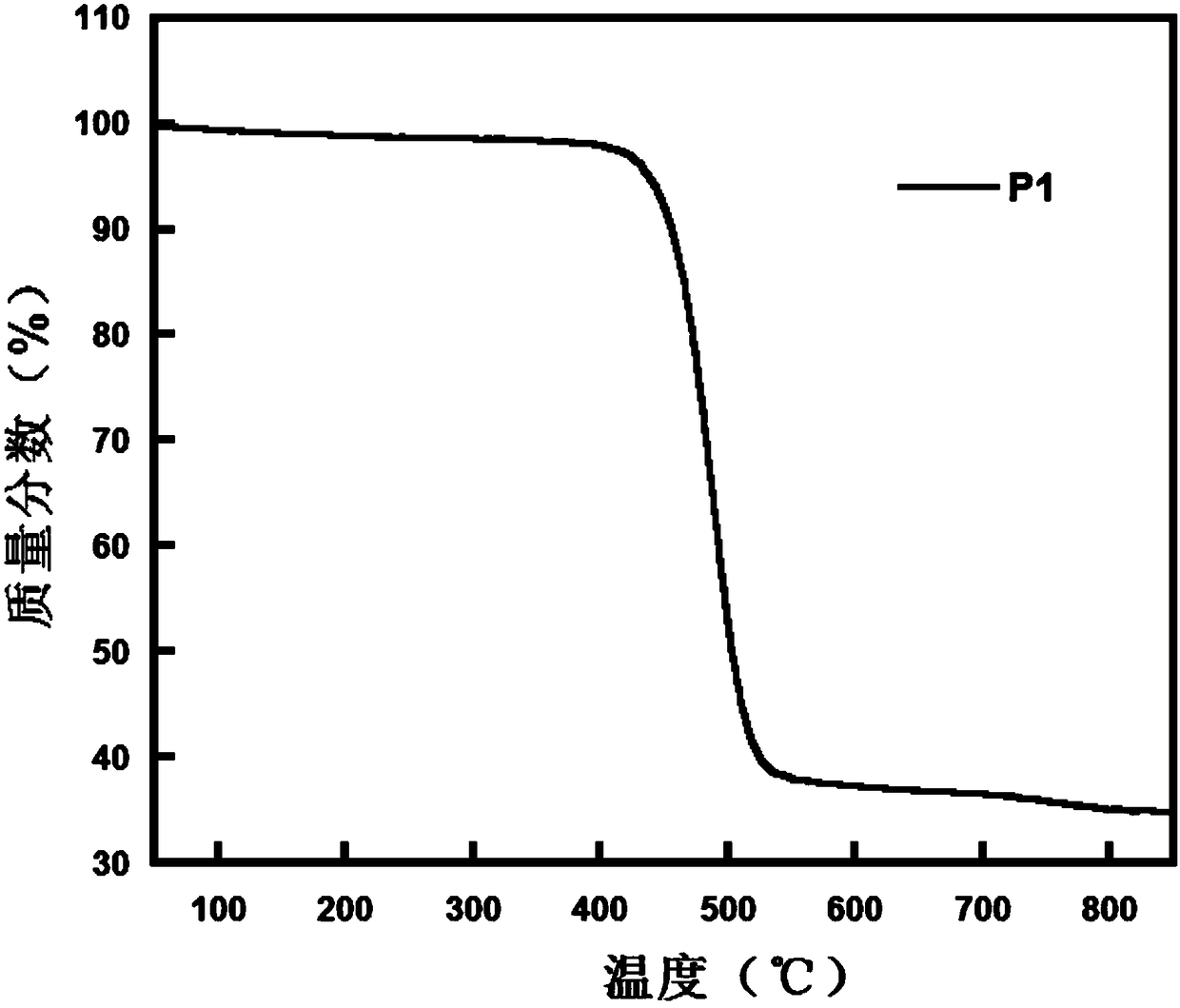
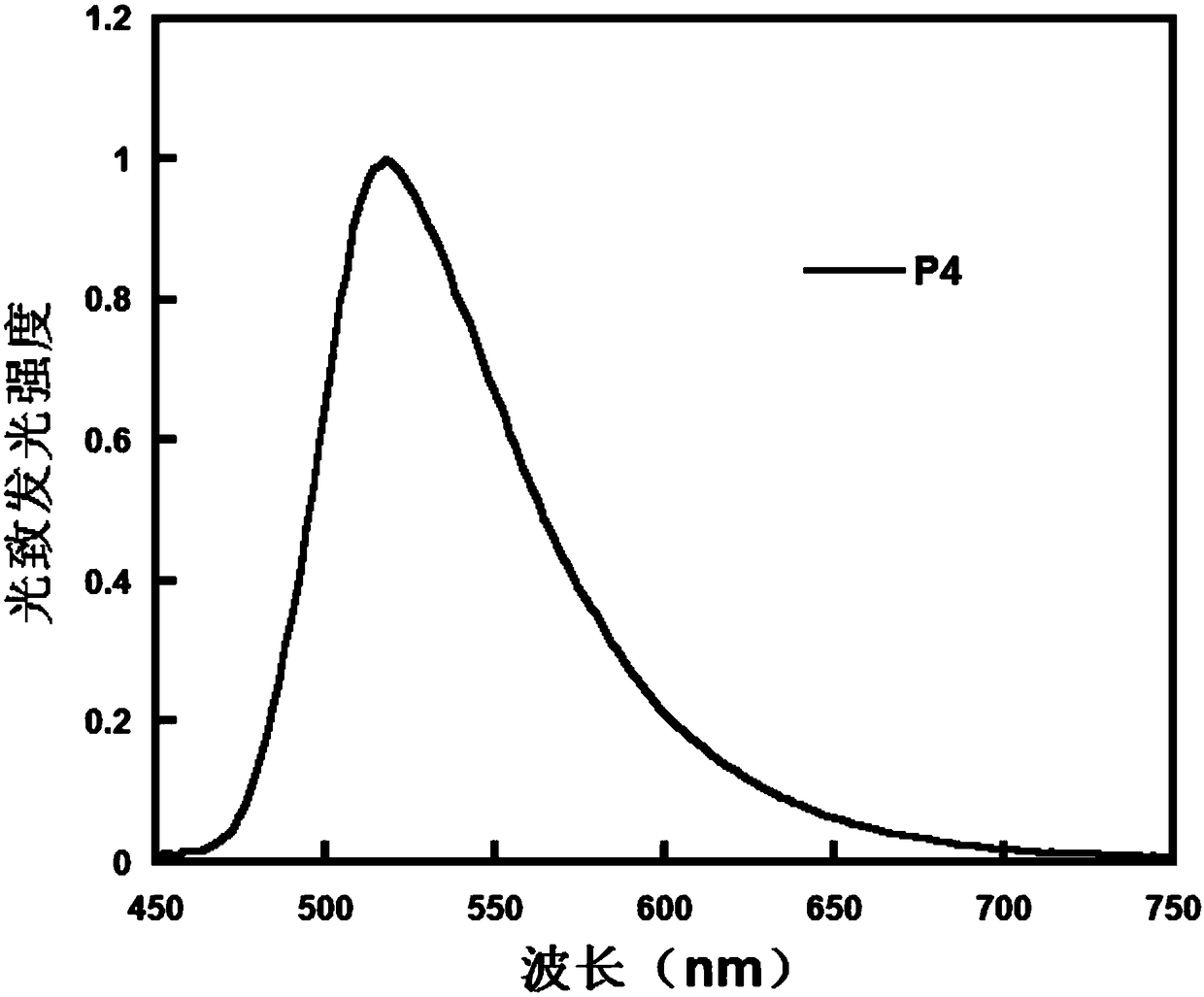
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of compound 4
[0052] (1) Preparation of compound 1
[0053] Under nitrogen protection, dissolve 3-bromo-9,10-phenanthrenequinone (2.87g, 10mmol) in 50mL of anhydrous tetrahydrofuran, stir and cool down to -78°C, then slowly add 2mol / L tetrahydrofuran of butylmagnesium bromide dropwise solution (30 mL, 60 mmol). After the dropwise addition was completed, it was naturally raised to room temperature and stirred overnight, and the reaction was quenched with a small amount of water. The product was extracted with dichloromethane after spin-drying THF, washed 3 times with saturated aqueous sodium chloride solution, and the solution in the organic phase was spin-dried to obtain 3.22 g of the product. Yield 80%. The results of MS and elemental analysis showed that the obtained compound was the target product.
[0054] (2) Preparation of Compound 2
[0055] Compound 1 (4.03 g, 10 mmol) was dissolved in 50 mL of acetic acid and 20 mL of trifluo...
Embodiment 2
[0062] Embodiment 2: the preparation of compound M1 and M2
[0063] (1) Preparation of Compound 5
[0064] Under nitrogen protection, methyl o-bromobenzoate (2.15g, 10mmol), pinacol diboronate (3.05g, 12mmol), potassium acetate (3.92g, 40mmol), [1,1'-bis(diphenyl Phosphino)ferrocene]palladium dichloride (0.49g, 0.5mmol) was added into 150mL of dioxane, heated to 85°C for 12 hours. Dioxane was removed by distillation under reduced pressure after the reaction was completed, the product was extracted with dichloromethane, washed three times with saturated aqueous sodium chloride solution, and dichloromethane was removed by distillation under reduced pressure, and the crude product was purified with petroleum ether:dichloromethane=3:1 (v / v) mixed solvent was used as eluent and purified by column chromatography to obtain 2.25 g of solid with a yield of 86%. 1 H NMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[00...
Embodiment 3
[0075] Embodiment 3: the synthesis of compound 11
[0076] The synthesis of compound 11 is similar to that of Example 1. According to the method of Example 1, the starting material was replaced by 2-bromo-9,10-phenanthrenequinone to prepare compounds 8, 9, 10, 11, 1 H NMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[0077] The chemical reaction equation for the synthesis of compounds 8-11 is as follows:
[0078]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com