Preparation method of multi-substituted pyrrole derivatives
A technology of pyrrole derivatives and multiple substitutions, applied in organic chemistry and other fields, can solve problems such as environmental pollution, high price, high safety risks, etc., and achieve the effects of reducing environmental pollution risks, reducing synthesis costs, and simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
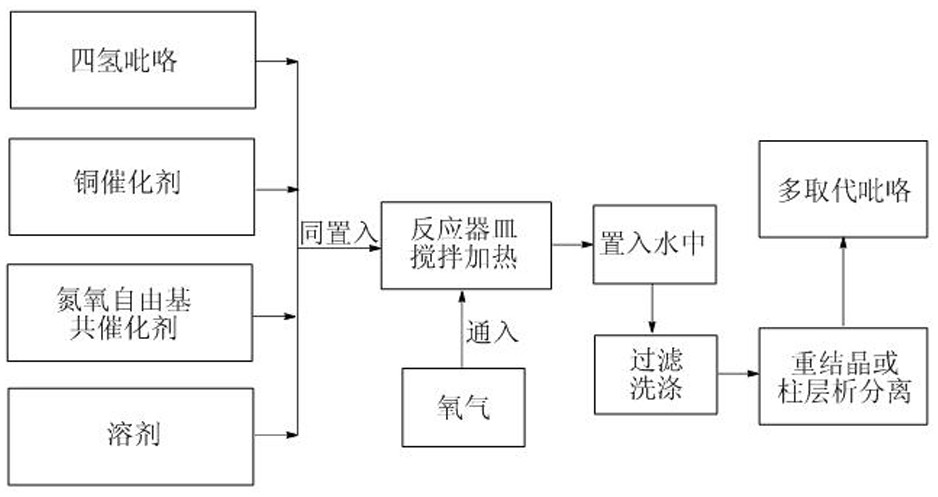
[0025] as attached figure 1 According to the technological process, take tetrahydropyrrole 5-(4-methoxyphenyl)-pyrrolidine-2,3,4-tricarboxylic acid methyl ester 70.3 mg (equivalent to 0.20 mmol), cuprous bromide 2.9 mg ( Equivalent to 0.02 mmol), 2,2,6,6-tetramethylpiperidine- N - 3.1 mg of oxygen free radicals (equivalent to 0.02 mmol) and 1.0 ml of 1,2-dichloroethane were added to the reaction vessel, and heated and stirred at 80 degrees Celsius for 5 hours under an oxygen atmosphere. plate) analysis until the reaction is complete. The mixture obtained above was poured into water, filtered, washed and dried to obtain a crude product. The crude product was separated by column chromatography to obtain 59.8 mg of the target polysubstituted pyrrole derivative of this example (86% yield).
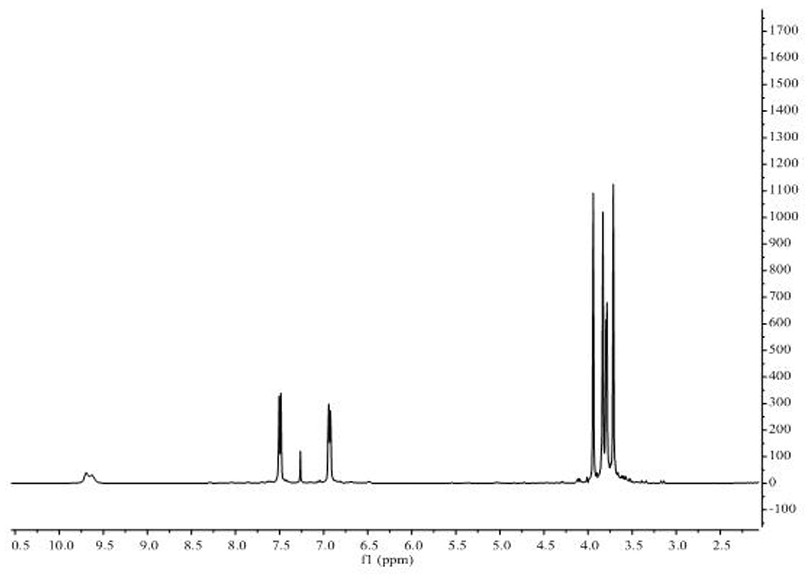
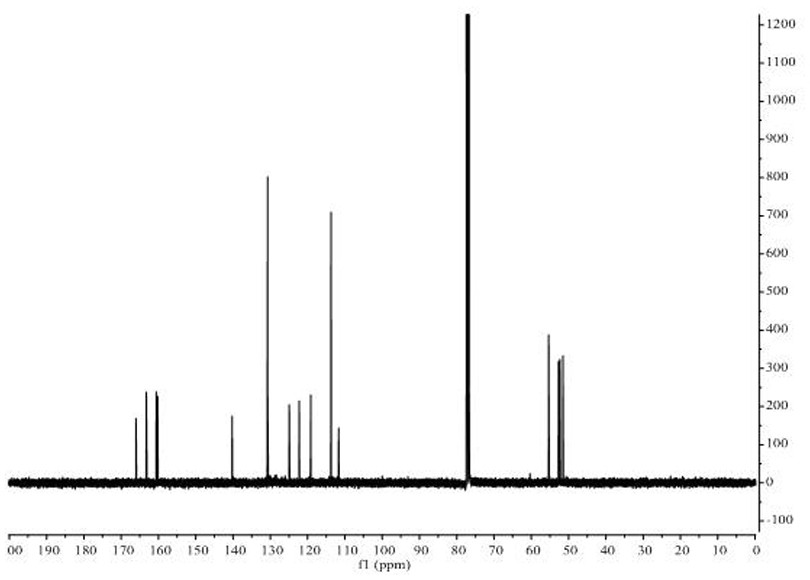
[0026] The target product in Example 1 was analyzed by a nuclear magnetic resonance spectrometer (model: AVANCE 400MHz, manufacturer: Bruker, Switzerland) to obtain figure 2 The H NMR spe...
Embodiment 2
[0029] as attached figure 1 The technical process of taking tetrahydropyrrole 5-cyclohexyl-3-phenyl-pyrrolidine-4-formic acid methyl ester-2-formic acid ethyl ester 71.9 mg (equivalent to 0.20 mmol), tetraacetonitrile cuprous hexafluorophosphate 3.7 mg (equivalent to 0.01 mmol), 4-acetamido-2,2,6,6-tetramethylpiperidine- N - 4.3 mg of oxygen free radicals (equivalent to 0.02 mmol) and 0.8 ml of benzene were added to the reaction vessel, heated and stirred at 90 degrees Celsius for 3 hours under an oxygen atmosphere, and analyzed by thin layer chromatography (TLC spot plate) until the reaction was complete . The mixture obtained above was poured into water, filtered, washed and dried to obtain a crude product. The crude product was separated by column chromatography to obtain 53.3 mg (yield: 75%) of the target product of this example, the multi-substituted pyrrole derivative.
Embodiment 3
[0031] as attached figure 1 The technological process of tetrahydropyrrole 3-methyl-5-pentyl-pyrrolidine-2,4-dicarboxylate 71.9 mg (equivalent to 0.20 mmol), copper acetate 5.4 mg (equivalent to 0.03 mmol) , 4-carbonyl-2,2,6,6-tetramethylpiperidine- N - 5.1 mg of oxygen free radicals (equivalent to 0.03 mmol) and 0.6 ml of ethyl acetate were added to the reaction vessel, heated and stirred at 80 degrees Celsius for 4 hours under an oxygen atmosphere, and analyzed by thin layer chromatography (TLC spot plate), until The response is complete. The mixture obtained above was poured into water, filtered, washed and dried to obtain a crude product. The crude product was separated by column chromatography to obtain 42.2 mg of the multi-substituted pyrrole derivative, the target product of this example (yield: 79%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com