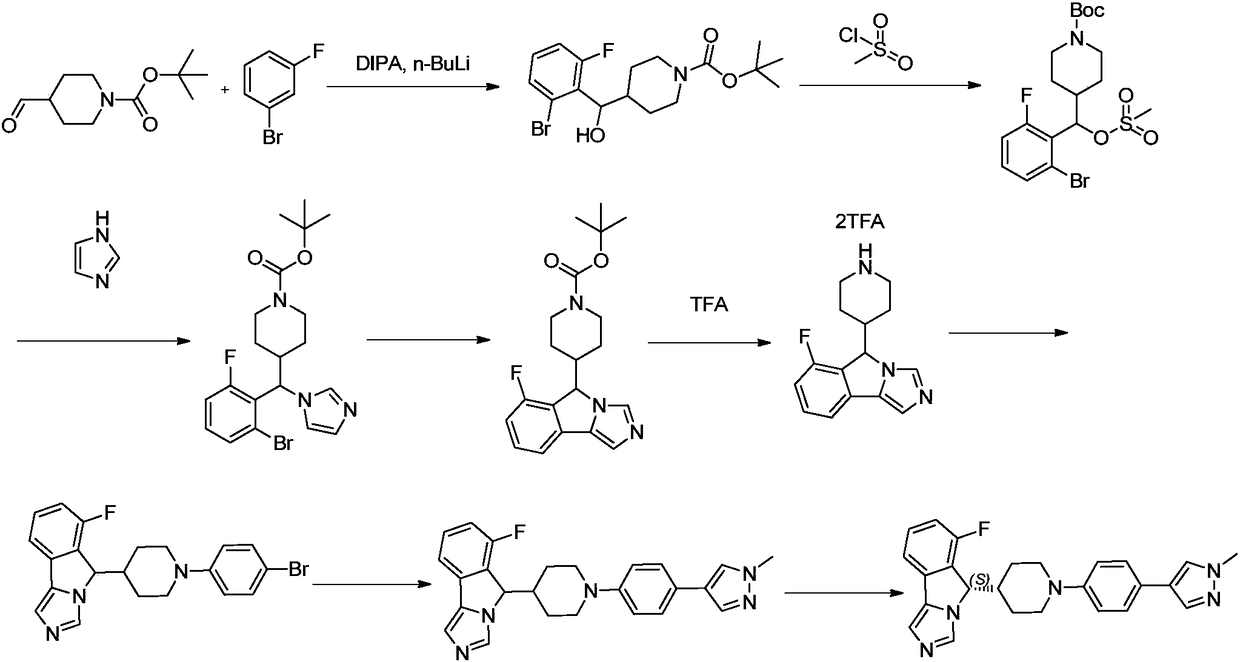
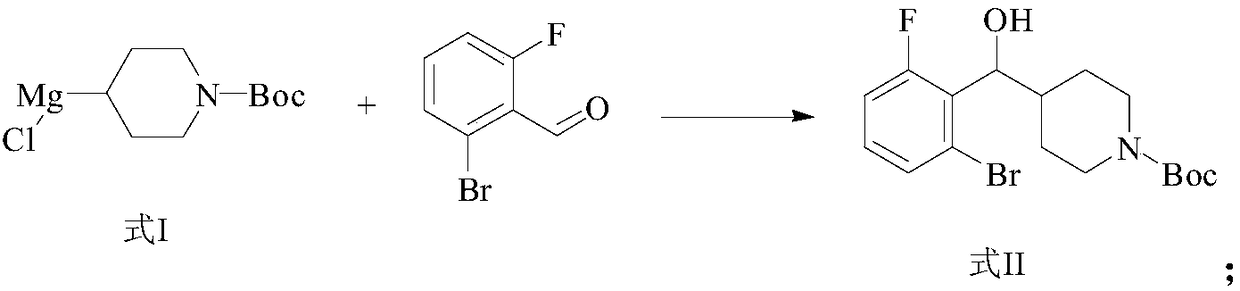
Method for preparing (S)-4-((2-bromo-6-fluoro-phenyl) hydroxymethyl) piperidine-1-tert-butyl formate
A technology of tert-butyl formate and hydroxymethyl, which is applied in the field of drug synthesis, can solve the problems of unsuitability for large-scale production, high cost, and high toxicity of reagents, and achieve the effects of large-scale production, mild conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In this example, (S)-4-((2-bromo-6-fluorophenyl)hydroxymethyl)piperidine-1-carboxylic acid tert-butyl ester was prepared by the following preparation method, and the preparation process was as follows:
[0048]
[0049] Concrete preparation method comprises the following steps:
[0050] (1) Preparation of tert-butyl 4-((2-bromo-6-fluorophenyl)(hydroxyl)methyl)piperidine-1-carboxylate
[0051] Prepare a solution of newly prepared Grignard reagent in tetrahydrofuran [from tert-butyl 4-bromopiperidine-1-carboxylate (75.5g, 286mmol), magnesium powder (13.9g, 572mol), a small amount of iodine particles, and tetrahydrofuran (300ml) at 50°C. Obtained] A solution of the aldehyde (58.06 g, 286 mmol) in THF was added slowly over 1 hour at 0°C, and the solution was allowed to warm to room temperature and stirred for 3 hours. Aqueous ammonium chloride solution was used to quench the reaction and extracted with dichloromethane, slurried with n-hexane (500ml) and filtered to obta...
Embodiment 2
[0057] (1) Preparation of tert-butyl 4-((2-bromo-6-fluorophenyl)(hydroxyl)methyl)piperidine-1-carboxylate
[0058] Prepare a fresh ether solution of Grignard reagent [from tert-butyl 4-bromopiperidine-1-carboxylate (75.5g, 286mmol), magnesium powder (13.9g, 572mol), a small amount of iodine particles, and ether (300ml) at 25°C Obtained] To a solution of the aldehyde (48.31 g, 238 mmol) in diethyl ether was slowly added at 0° C. over 1.5 hours, and the solution was allowed to warm to room temperature and stirred for 8 hours. Aqueous ammonium chloride solution was used to quench the reaction and extracted with dichloromethane. The product (80.39 g, yield 87%) was obtained by beating with petroleum ether (500 ml) and filtered. MS = 388.3. 1 H-NMR (400MHz, CDCl3): δ (ppm) 7.36-7.34 (d, J = 7.6Hz, 1H, Ar-H), 7.14-7.08 (dd, J = 13.8Hz, J = 8.6Hz, 1H, ArH ),7.05-7.00(m,1H,Ar-H),4.87(s,1H,CH),4.18-4.04(m,2H,CH 2 ), 2.68-2.55 (m, J=9.4Hz, 2H, CH 2 ),2.46(s,1H,CH),2.15-2.08(m,2H,CH ...
Embodiment 3
[0065] (1) Preparation of tert-butyl 4-((2-bromo-6-fluorophenyl)(hydroxyl)methyl)piperidine-1-carboxylate
[0066] The 2-methyltetrahydrofuran solution of the newly prepared Grignard reagent [from tert-butyl 4-bromopiperidine-1-carboxylate (75.5g, 286mmol), magnesium powder (13.9g, 572mol), a small amount of iodine particles, 2-methyl Prepared by heating tetrahydrofuran (300ml) at 50°C] at 5°C for 0.5 hours was slowly added to a solution of aldehyde (29.03g, 143mmol) in 2-methyltetrahydrofuran, the solution was warmed to room temperature and stirred for 1 hour. Aqueous ammonium chloride solution was used to quench the reaction and extracted with dichloromethane, slurried with n-hexane (500ml) and filtered to obtain the product (49.41g, yield 89.0%). MS = 388.4. 1 H-NMR (400MHz, CDCl3): δ (ppm) 7.38-7.36 (d, J = 7.6Hz, 1H, Ar-H), 7.16-7.10 (dd, J = 13.8Hz, J = 8.6Hz, 1H, ArH ),7.07-7.02(m,1H,Ar-H),4.89(s,1H,CH),4.121-4.06(m,2H,CH 2 ),2.70-2.57(m, J=9.4Hz, 2H, CH 2 ),2.48(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com