Method for producing iodine product through electronic control ionic membrane extraction coupling electrolysis process
A technology of ionic membrane and electrolysis method, applied in electrolysis process, electrolysis components, electrodes, etc., can solve the problems of reduced reuse effect, waste of resources, low iodine production, etc., to improve adsorption and desorption efficiency, eliminate secondary pollution, The effect of improving adsorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
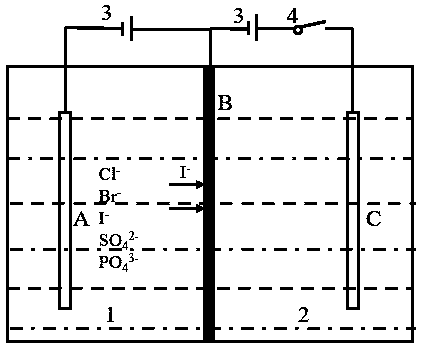
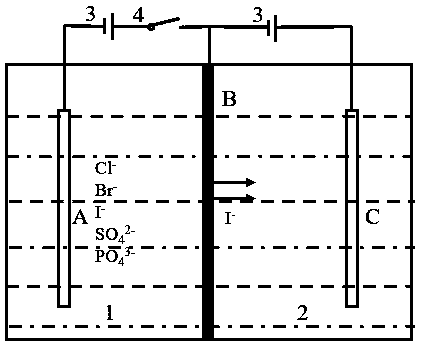
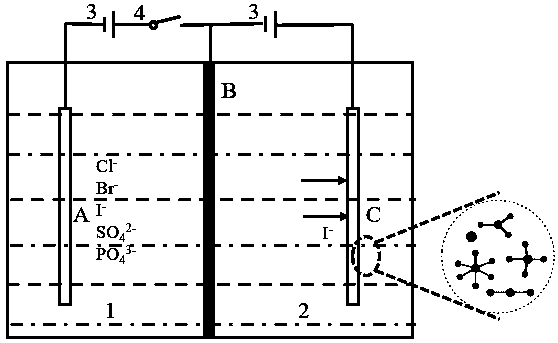
[0038] An iodine-containing solution in chamber A: the solubility of iodide ions is 30 mg / L, and the volume of the solution is 200 mL. Iodine ion-doped polypyrrole is used as the electroactive extraction material. The mass of the extracted material is 0.0162g. A voltage of 0.5V is applied to the membrane electrode for 6 hours of adsorption. The UV spectrophotometer is used to detect the iodide ion in the solution in chamber A. The adsorption capacity reached 4.8mg. After 6 hours of membrane extraction and adsorption stage, the adsorption capacity of iodide ions has reached saturation. Turn off the switch in chamber A and turn on the switch in chamber B. In chamber B, there is 200 mL of 10 mg / L potassium iodide solution. A voltage of 0.5V is applied to the membrane electrode, and a reduction reaction occurs on the membrane electrode to realize the stripping process. After a 2h stripping phase, the content of iodide ion detected in the solution of chamber B is 6.7mg, and the maxi...
Embodiment 2
[0040] An iodine-containing solution in chamber A: the solubility of iodide ions is 20 mg / L, and the volume of the solution is 200 mL. Iodine ion-doped polypyrrole is used as the electroactive extraction material. The mass of the extracted material is 0.0122g. A voltage of 0.5V is applied to the membrane electrode for 6 hours of adsorption. The UV spectrophotometer is used to detect the iodide ion in the solution in chamber A. The adsorption capacity reached 3.5mg. After 6 hours of membrane extraction and adsorption stage, the adsorption capacity of iodide ions has reached saturation. Turn off the switch in chamber A and turn on the switch in chamber B. In chamber B, there is 200 mL of 10 mg / L potassium iodide solution. A voltage of 0.5V is applied to the membrane electrode, and a reduction reaction occurs on the membrane electrode to realize the stripping process. After a 2h stripping stage, the content of iodide ion detected in the solution of chamber B is 5.4mg, and the maxi...
Embodiment 3
[0042] An iodine-containing solution in chamber A: the solubility of iodide ions is 30 mg / L, and the volume of the solution is 200 mL. Iodine ion-doped polypyrrole was used as the electroactive extraction material. The mass of the extracted material was 0.0159g. A voltage of 0.5V was applied to the membrane electrode for 6 hours of adsorption. The UV spectrophotometer was used to detect the iodide ion in the solution in chamber A. The adsorption capacity reached 4.6mg. After 6 hours of membrane extraction and adsorption stage, the adsorption capacity of iodide ions has reached saturation. Turn off the switch in chamber A and turn on the switch in chamber B. In chamber B, there is 200 mL of 10 mg / L potassium iodide solution. A voltage of 0.9V is applied to the membrane electrode, and a reduction reaction occurs on the membrane electrode to realize the stripping process. After a 2h stripping stage, the content of iodide ion detected in the solution of chamber B is 0.5mg, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com