Preparation method of lithium iron phosphate positive electrode material precursor
A positive electrode material, lithium iron phosphate technology, applied in the field of lithium ion battery cathode material preparation, can solve the problems of poor uniformity, high energy consumption, long grinding time of lithium iron phosphate precursor, etc., achieve small particle size, save cost, and reduce synthesis Effects of Time and Energy Consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
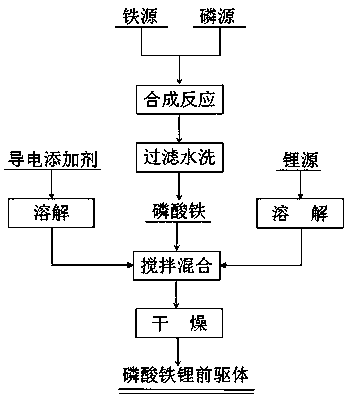
[0025] Such as figure 1 As shown, the preparation method of the lithium iron phosphate positive electrode material precursor, its specific steps are as follows:
[0026] Step 1. Mix iron source (iron powder) and phosphorus source (phosphoric acid, concentration 40wt%) according to the molar ratio of Fe:P 1:1 and then prepare a solution. Control the pH value of the solution to 2, and add 1.3 moles of Fe Double hydrogen peroxide, combined reaction at 60°C for 2 hours, aged at room temperature for 4 hours to obtain ferric phosphate precipitate, washed the ferric phosphate precipitate with water until the pH value of the filtered water was 7-8, and dried to obtain ferric phosphate powder;
[0027] Step 2. The lithium source (lithium carbonate) is prepared into a lithium source solution according to the molar ratio of Li:Fe:P in the iron source and phosphorus source of 1.03:1:1; the lithium source is 1:25g / mL according to the solid-liquid ratio Add deionized water, then pass in ca...
Embodiment 2
[0032] Such as figure 1 As shown, the preparation method of the lithium iron phosphate positive electrode material precursor, its specific steps are as follows:
[0033] Step 1. Mix iron source (ferrous sulfate heptahydrate) and phosphorus source (ammonium dihydrogen phosphate) according to the molar ratio of Fe:P 1:1, and add deionized water according to the solid-liquid ratio of 1:5g / mL to form Iron source solution and phosphorus source solution, drop the phosphorus source solution into the iron source solution, control the pH value of the solution to 2, add hydrogen peroxide with 1.3 times the molar number of Fe, and react at a temperature of 70°C for 2 hours, and age at room temperature After 2 hours, ferric phosphate precipitate was obtained, and the ferric phosphate precipitate was washed until the pH value of the filtered water was 7-8, and dried to obtain ferric phosphate powder;
[0034] Step 2. The lithium source (lithium hydroxide) is prepared into a lithium source...
Embodiment 3
[0039] Such as figure 1 As shown, the preparation method of the lithium iron phosphate positive electrode material precursor, its specific steps are as follows:
[0040] Step 1. Iron source (a mixture of ferrous sulfate heptahydrate and ferrous oxalate with a mass ratio of 1:1) and a phosphorus source (a mixture of ammonium dihydrogen phosphate and diammonium hydrogen phosphate with a mass ratio of 1:1) according to Fe: The molar ratio of P is 1:1, and deionized water is added according to the solid-liquid ratio of 1:10g / mL to form iron source solution and phosphorus source solution. The phosphorus source solution is added dropwise to the iron source solution, and the pH of the solution is controlled to be 2. Add hydrogen peroxide with 1.2 times the molar number of Fe, react at 70°C for 2 hours, and age at room temperature for 4 hours to obtain iron phosphate precipitation, wash the iron phosphate precipitation until the pH value of the filtered water is 7-8, and dry to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com