Preparation method of microhydrogel capable of simultaneously loading and releasing hydrophilic and hydrophobic drugs
A hydrophobic drug, hydrophilic and hydrophobic technology, applied in the field of preparation of micro-hydrogels, can solve the problems of complicated synthesis process, affecting a wide range of use, etc., and achieve the effects of reducing cytotoxicity, prolonging administration period, and improving therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
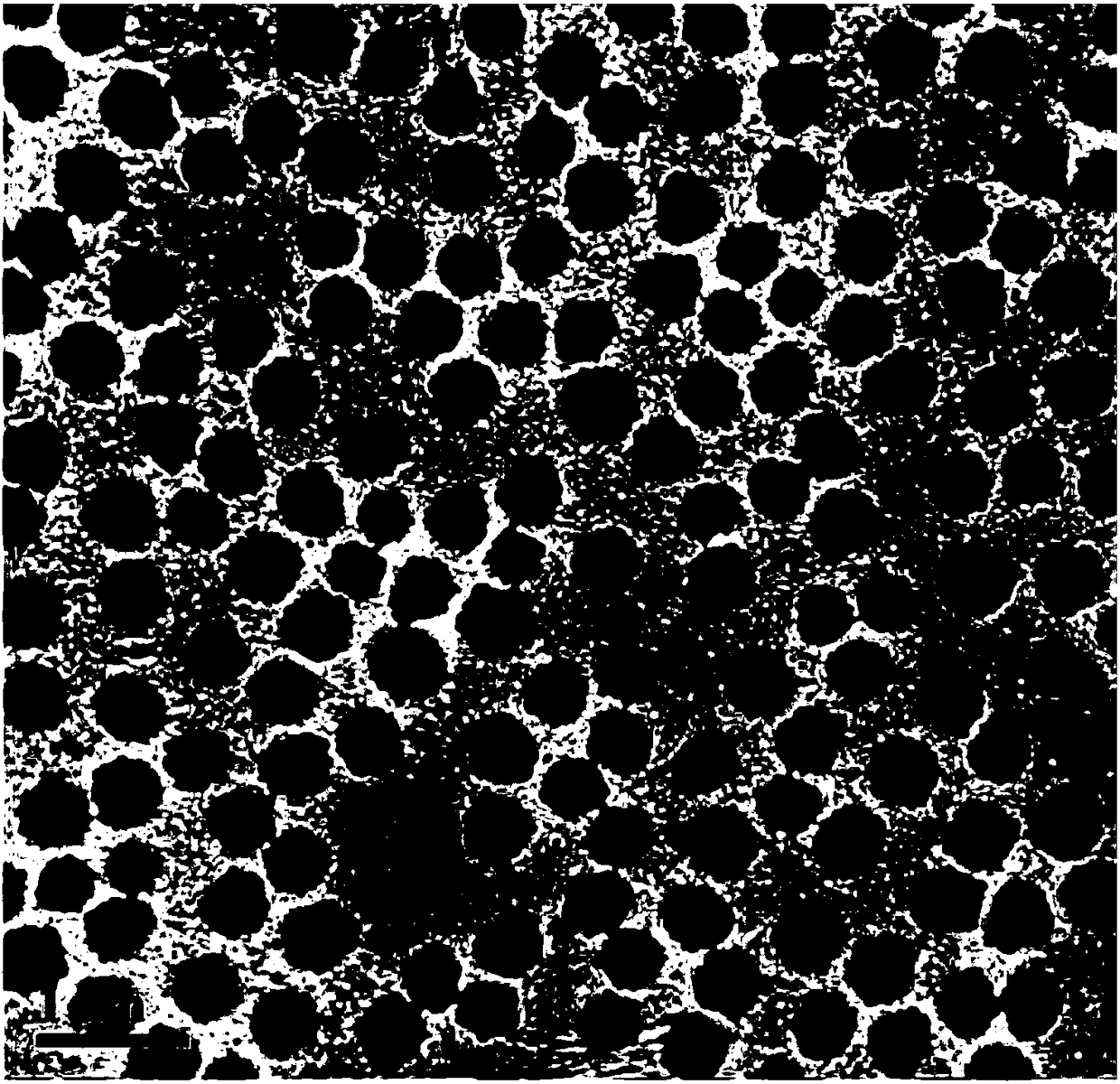
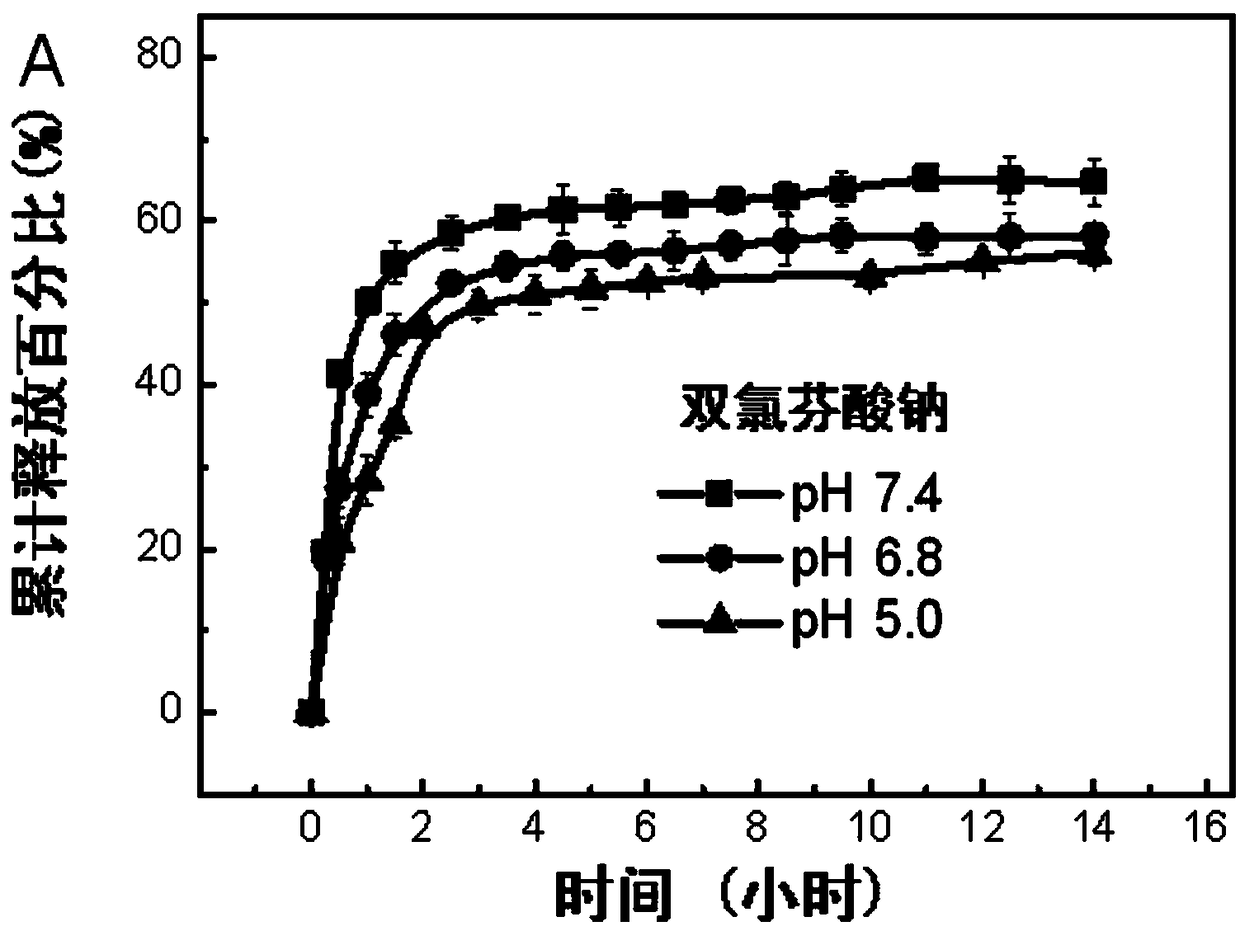
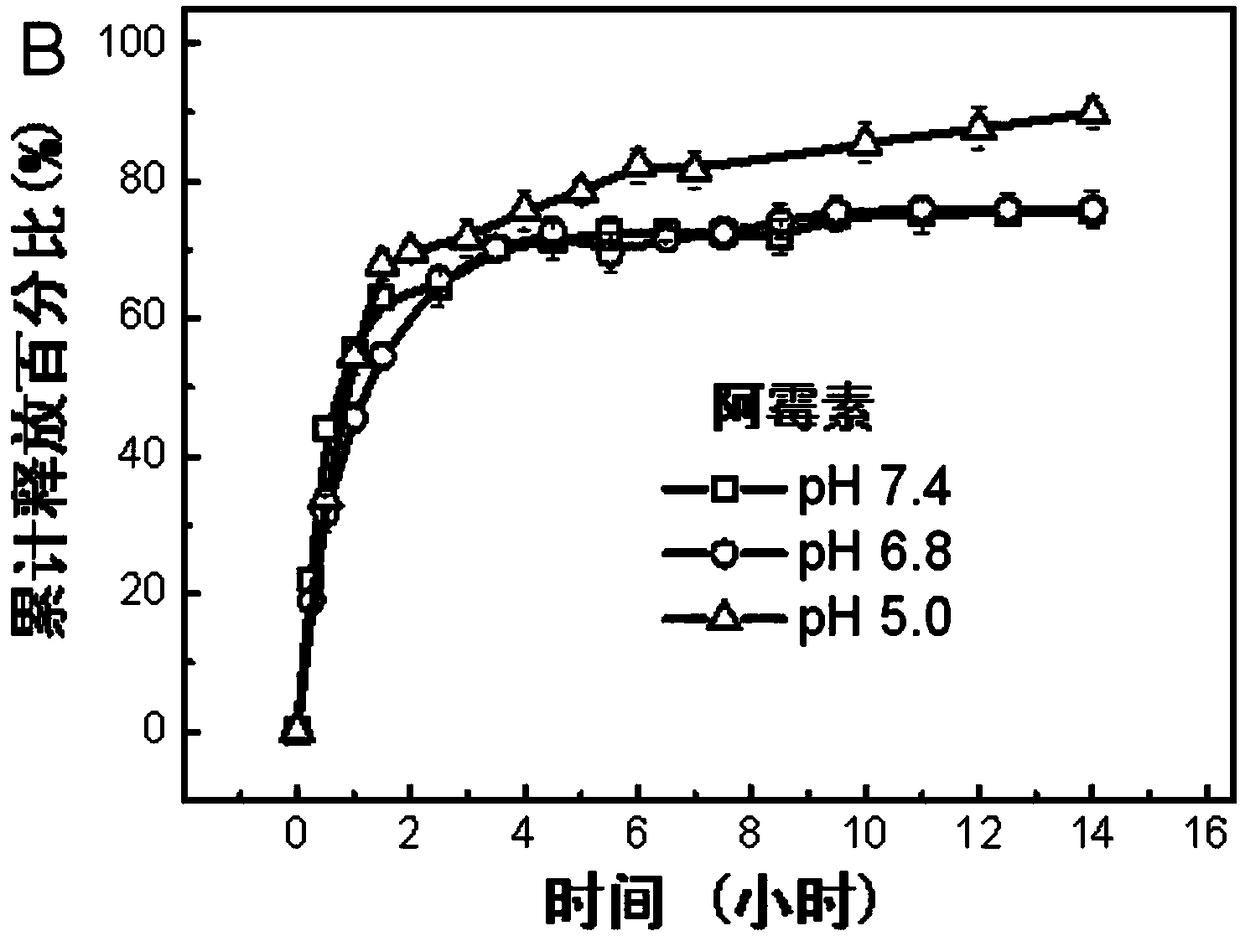
Image
Examples
Embodiment 1
[0060] Hydroxyethyl methacrylate (1.3 g, 10 mmol) and sodium carbonate (2.1 g, 20 mmol) were dissolved in 40 ml of dichloromethane, and cooled to 0° C. in an ice bath. Weigh 4-dimethylaminopyridine (24 mg, 0.2 mmol) into the reaction flask, dissolve cinnamoyl chloride (0.34 g, 2 mmol) in 10 ml of dichloromethane, and slowly add it dropwise to the above solution with a constant pressure dropping funnel middle. After the dropwise addition, the ice bath was removed, and the reaction was stirred at room temperature for 24 hours. After the reaction was completed, sodium carbonate was removed by vacuum water pump filtration to obtain a colorless liquid. The colorless liquid was washed three times with 50 ml of saturated sodium chloride solution, separated to remove the inorganic phase, and the obtained organic layer was dried overnight with anhydrous magnesium sulfate to remove water. Magnesium sulfate was removed by vacuum water pump filtration, and dichloromethane was removed by...
Embodiment 2
[0063] Hydroxyethyl methacrylate (1.3 g, 10 mmol) and sodium carbonate (2.1 g, 20 mmol) were dissolved in 40 ml of dichloromethane and cooled to °C in an ice bath. Weigh 4-dimethylaminopyridine (24 mg, 0.2 mmol) into the reaction flask, dissolve cinnamoyl chloride (1.7 g, 10 mmol) in 10 ml of dichloromethane, and slowly add it dropwise to the above solution with a constant pressure dropping funnel middle. After the dropwise addition, the ice bath was removed, and the reaction was stirred at room temperature for 24 hours. After the reaction was completed, sodium carbonate was removed by vacuum water pump filtration to obtain a colorless liquid. The colorless liquid was washed three times with 50 ml of saturated sodium chloride solution, separated to remove the inorganic phase, and the obtained organic layer was dried overnight with anhydrous magnesium sulfate to remove water. Magnesium sulfate was removed by vacuum water pump filtration, and dichloromethane was removed by rot...
Embodiment 3
[0066] Hydroxyethyl acrylate (1.3 g, 10 mmol) and sodium carbonate (3.1 g, 30 mmol) were dissolved in 40 ml of dichloromethane and cooled to °C in an ice bath. Weigh 4-dimethylaminopyridine (24 mg, 0.2 mmol) into the reaction flask, dissolve cinnamoyl chloride (3.4 g, 20 mmol) in 10 ml of dichloromethane, and slowly add it dropwise to the above solution with a constant pressure dropping funnel middle. After the dropwise addition, the ice bath was removed, and the reaction was stirred at room temperature for 24 hours. After the reaction was completed, sodium carbonate was removed by vacuum water pump filtration to obtain a colorless liquid. The colorless liquid was washed three times with 50 ml of saturated sodium chloride solution, separated to remove the inorganic phase, and the obtained organic layer was dried overnight with anhydrous magnesium sulfate to remove water. Magnesium sulfate was removed by vacuum water pump filtration, and dichloromethane was removed by rotary ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com