Method for preparing RGD-polypeptide grafted poly(maleidohexanediamine acid-D,L-lactic acid)/beta-TCP composite material
A technology of adipamide acid and porous composite materials, applied in medical science, prostheses, etc., can solve the problems of loss of mechanical strength of matrix materials, excessive molecular weight drop, and different material properties, so as to achieve controllable reactions and products, The modification process is simple and the effect of improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) First, dissolve 2.335g of hexamethylenediamine in 10mL of dichloromethane, then add 6.106mL of triethylamine and stir evenly; dissolve 1.6335g of maleic anhydride in 5mL of dichloromethane. Under the condition of stirring at room temperature, the dichloromethane solution of maleic anhydride was added dropwise to the dichloromethane solution of hexamethylenediamine within 0.5h, and the reaction was continued at room temperature for 2h after the dropwise addition was completed. After the reaction is completed, the obtained mixed solution is separated by a THF-absolute ethanol co-precipitation method to obtain the maleamide hexamethylene diamine monomer, which is vacuum-dried for future use. The specific operation of the THF-absolute ethanol co-precipitation method is as follows: dissolve the reacted substance in an appropriate amount of THF, then drop excess absolute ethanol into the mixed solution, and collect the precipitate.
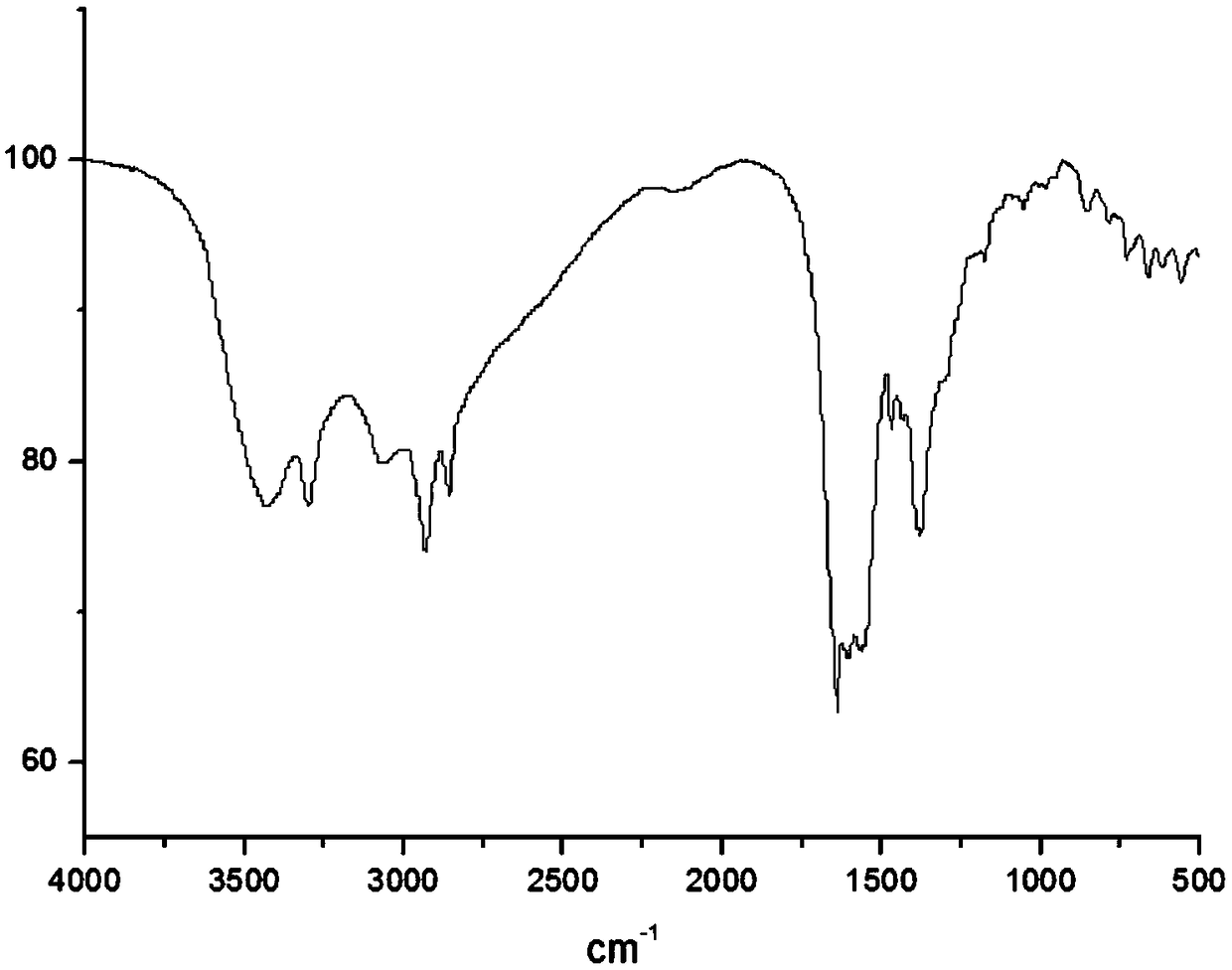
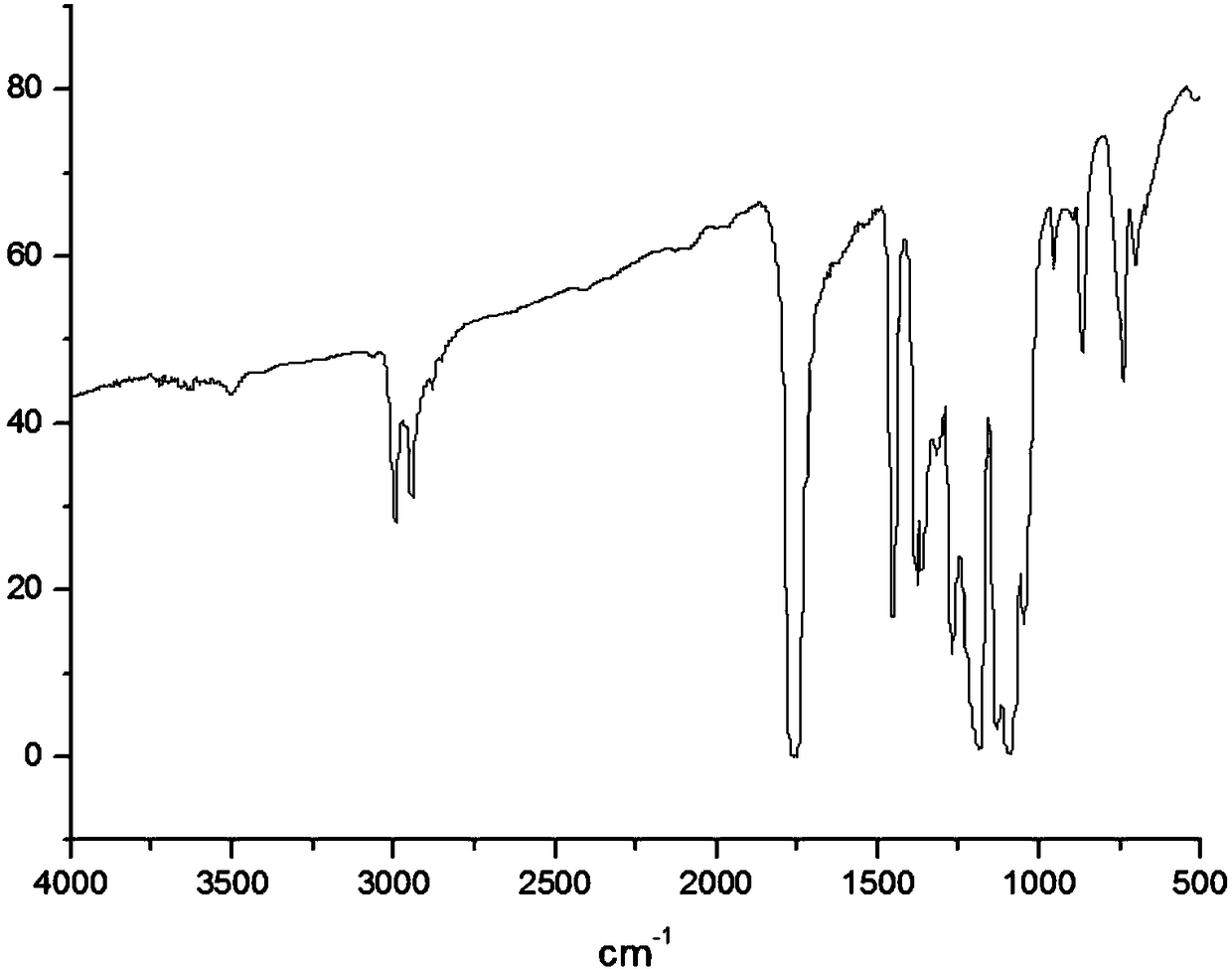
[0029] The infrared spectrogram of the ...
Embodiment 2
[0037] 1) First, dissolve 2.365g of hexamethylenediamine in 10mL of dichloromethane, then add 4.64mL of triethylamine and stir evenly; dissolve 1.25g of maleic anhydride in 5mL of dichloromethane. Under the condition of stirring at room temperature, the dichloromethane solution of maleic anhydride was added dropwise to the dichloromethane solution of hexamethylenediamine within 0.5h, and the reaction was continued at room temperature for 2h after the dropwise addition was completed. After the reaction is completed, the obtained mixed solution is separated by a THF-absolute ethanol co-precipitation method to obtain the maleamide hexamethylene diamine monomer, which is vacuum-dried for future use.
[0038] 2) Dissolve 5g of poly-D,L-lactide in an appropriate amount of tetrahydrofuran, and dissolve 0.504g of maleic hexamethylene diamic acid in an ethanol solution with a volume fraction of 90%. Under stirring conditions, drop the ethanol solution of maleic hexamethylene diamic aci...
Embodiment 3
[0042] 1) First, dissolve 2.5g of hexamethylenediamine in 10mL of dichloromethane, then add 5.9mL of triethylamine and stir evenly; dissolve 1.32g of maleic anhydride in 5mL of dichloromethane. Under the condition of stirring at room temperature, the dichloromethane solution of maleic anhydride was added dropwise to the dichloromethane solution of hexamethylenediamine within 0.5h, and the reaction was continued at room temperature for 2h after the dropwise addition was completed. After the reaction is completed, the obtained mixed solution is separated by a THF-absolute ethanol co-precipitation method to obtain the maleamide hexamethylene diamine monomer, which is vacuum-dried for future use.
[0043] 2) Dissolve 5g of poly-D,L-lactide in an appropriate amount of tetrahydrofuran, and dissolve 0.504g of maleic hexamethylene diamic acid in an ethanol solution with a volume fraction of 90%. Under stirring conditions, drop the ethanol solution of maleic hexamethylene diamic acid i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com