Dibenzoanthracene oxepanone compound and preparation method and application thereof
A technology of compounds and microorganisms, applied in the field of compounds, to achieve high-efficiency anti-Vibrio parahaemolyticus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 2-(1-hydroxy-n-pentyl)benzoic acid (III):
[0042] Dissolve 1.24g (6.5mmol) NBP in 10mL of methanol, add 10mL of 2M NaOH solution, reflux and stir for 0.5h, distill methanol off under reduced pressure, add 10mL of distilled water to dilute, cool to -5°C, and use 5% dilute hydrochloric acid under vigorous stirring Acidify to pH 2-3, extract with ether (15mL×3), and directly put into the next reaction without any purification.
Embodiment 2
[0044] Synthesis of 2-(1-acetyl n-pentyl)benzoic acid:
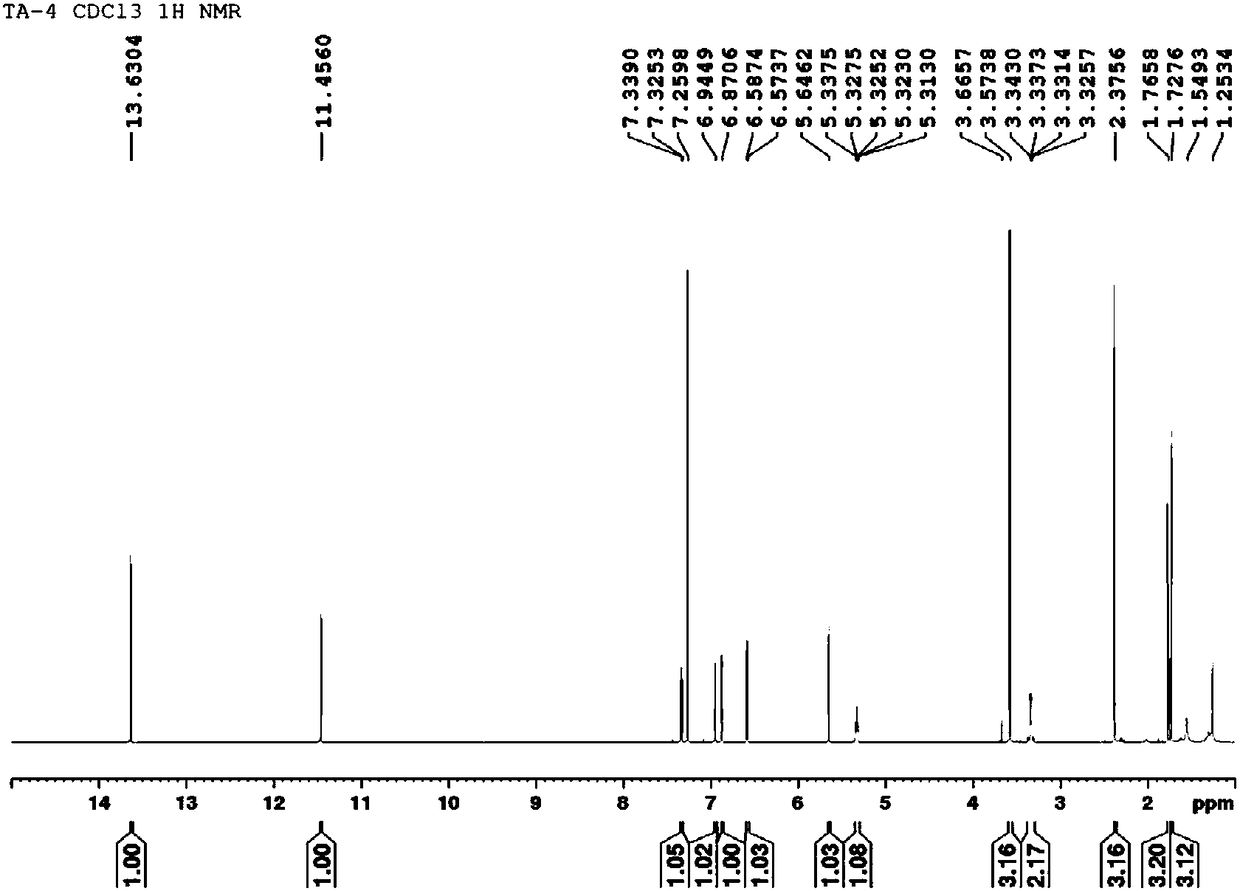
[0045] Dilute the ether solution containing III above with 200mL of dichloromethane, add 2.7mL (19.6mmol) of triethylamine and 0.5g of DMAP respectively, and add 1.4mL (19.6mmol) of acetyl chloride dropwise at -10°C. Stir at ℃ for 5h, add 10mL of water, stir at room temperature for 0.5h, separate the organic layer, Na 2 SO 4 Dry, filter, and concentrate to obtain a waxy solid, which is recrystallized from n-hexane to obtain 1.06 g of white needle-like crystals, with a yield of 65%. mp 65-66℃.MS(ESI):m / z 249.1[M -H]-. 1 H NMR (300MHz, CDCl 3 ):δ0.93(t,3H,CH 3 ,J=8.5Hz),1.37–1.42(m,4H, 2×CH 2 ), 1.88–1.91 (m,2H,CH 2 ),2.13–2.33(m,3H,COCH 3 ),6.61–6.72(m,1H,OCHCH 2 ), 7.37–7.40(m,1H,ArH),7.56–7.62(m,2H,ArH),8.05(d,1H,ArH,J=8.1Hz),10.98(brs, 1H,COOH). 13 C NMR (75MHz, CDCl 3 ): δ172.0, 166.5, 140.8, 133.1, 130.3, 130.0, 127.1, 125.7, 74.8, 41.0, 36.3, 27.8, 22.4, 13.8.
Embodiment 3
[0047] A representative intermediate V for the coupling of diols based on 2-(1-acetyl-n-pentyl)benzoic acid 1 Synthesis:
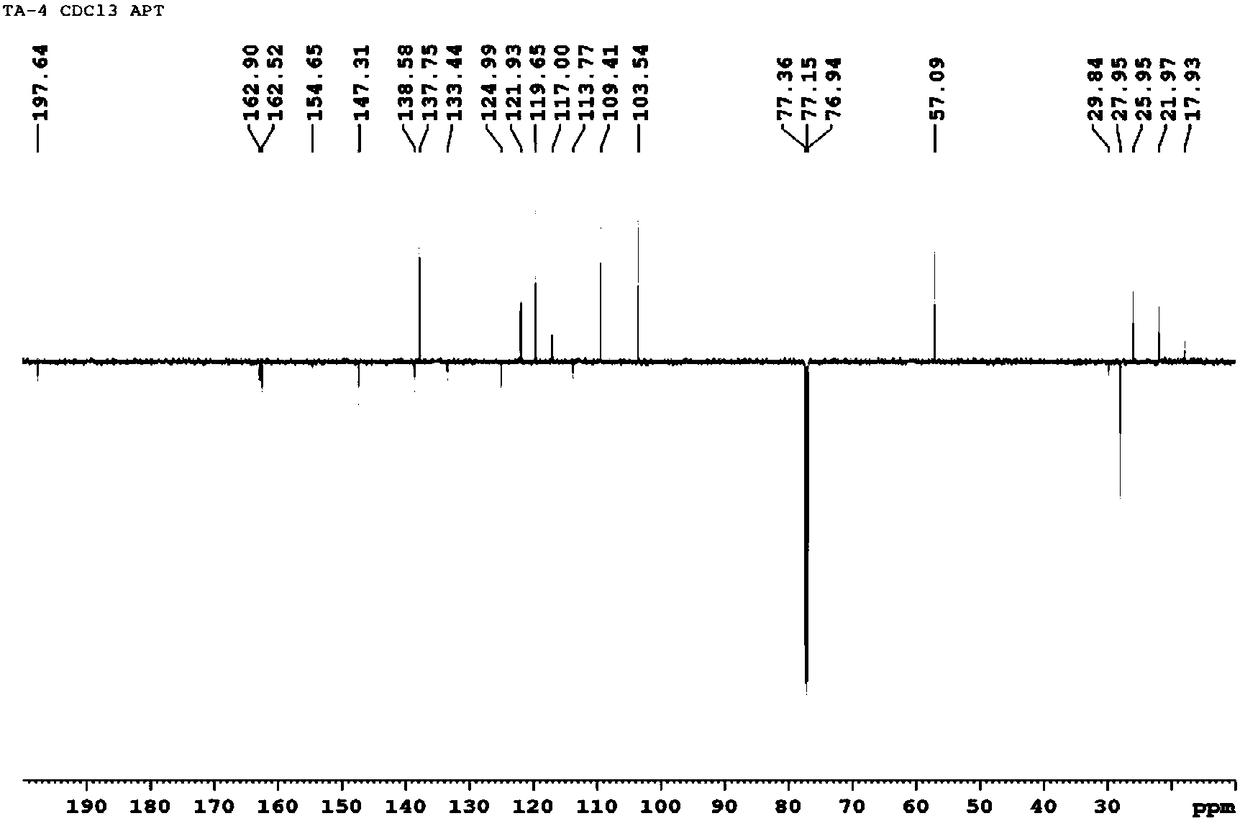
[0048] Dissolve 2-(1-acetyl n-pentyl)benzoic acid (2.50g, 10.0mmol) in anhydrous dichloromethane (50mL), add EDAC (2.29g, 12.0mmol) and a catalytic amount of DMAP, stir at room temperature for 0.5 h, then added ethylene glycol (0.62g, 10.0mmol), stirred at room temperature for 5h, filtered, concentrated under reduced pressure, and obtained oily substance 1.71 through column chromatography [petroleum ether: ethyl acetate (v:v) = 30:1] g, yield 58%. MS(ESI):m / z 317.1[M+Na] + . 1 H NMR (300MHz, CDCl 3 ):δ0.807(t, 3H,CH 3 ,J=7.0Hz), 1.181-1.356(m, 4H, 2×CH 2 ),1.730-1.777(m,2H,CH 2 ),1.965(s,3H, COCH 3 ),3.823-3.862(m,2H,CH 2 ),4.269-4.474(m,2H,CH 2 ),5.206(s,1H,OH),6.452(t,1H,COOCH,J=6.7Hz),7.197-7.265(m,1H,ArH),7.441-7.444(m,2H,ArH),7.750-7.777 (m,1H,ArH). 13 C NMR (75MHz, CDCl 3 ): δ170.90, 167.51, 142.37, 132.15, 129.94, 129.34, 127.39, 126.46...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com