Recombinant bacterium of lipase expressing R configuration selectivity and application thereof
A selective and recombinant bacteria technology, applied in the direction of enzymes, bacteria, hydrolytic enzymes, etc., can solve the problems of different stereoselective catalytic functions, complex processes, high costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Discovery of R-configuration-selective lipase AflB
[0031] Through the mining of the protein database, an Aspergillus fumigatus Af293lipase (GenBank: XP_749106) amino acid sequence was found, and it was found that the enzyme showed a low sequence identity with CalB, a lipase widely used in industry. Identity=31%, and contained a CalB that did not have The N-terminal alpha-helical domain. The N-terminal α-helical domain is favorable for the binding of R-configuration naproxen ((-)-2-(6-methoxy-2-naphthyl)propionic acid) through homology modeling and substrate molecular docking . And on the basis of the molecular docking, the corresponding amino acid residues in the substrate pocket were replaced by computer simulation, and Aspergillus fumigatus Af293lipase was further modified to make it more inclined to bind R-configuration naproxen. Finally, the modified Aspergillus fumigatus Af293lipase was named R-configuration-selective lipase AflB, which consisted of 438 amino a...
Embodiment 2
[0034] Expression and Purification of R Configuration Selective Lipase AflB
[0035] 1. Construction of recombinant plasmids
[0036] The lipase AflB coding gene sequence is shown in SEQ ID NO.2. The lipase AflB gene was obtained through total gene synthesis, and then cloned into the pET28a plasmid to obtain the plasmid pET28a-AflB.
[0037] 2. Obtaining of lipase AflB producing bacteria with recombinant R configuration selectivity
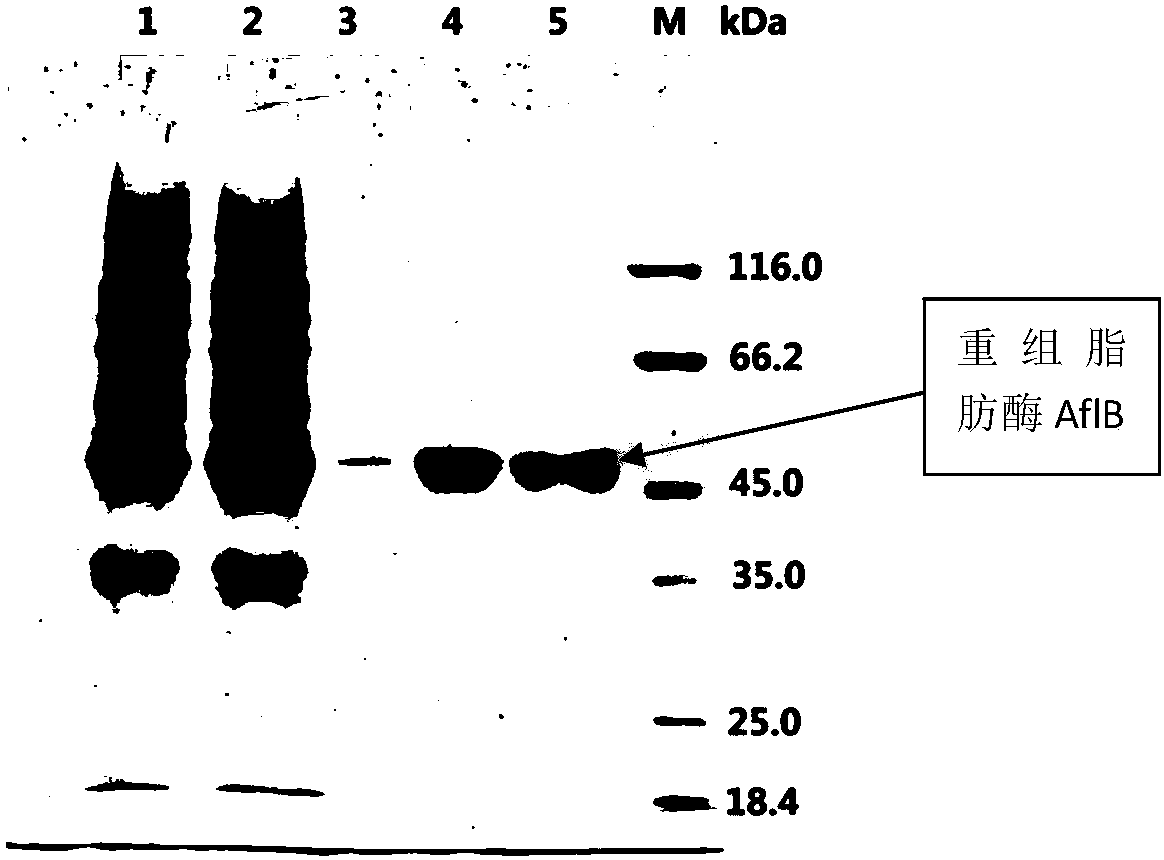
[0038] Take a tube of Escherichia coli SHuffle T7Competent E.coli (Cat.C3026, NEB) competent cells, thaw on ice, transfer 50ul to a pre-cooled 1.5ml centrifuge tube with a pre-cooled pipette tip, and then add the recombinant Plasmid pET28a-AflB 1ul, shake gently to mix, place in ice bath for 30 minutes. Quickly transfer to a 42°C water bath for exactly 40 seconds. Quickly transfer to ice and chill for 2 minutes. Add 500ul LB culture medium to each tube and incubate at 37°C for 45 minutes. Spread 20ul on a plate containing antibiotics (50mg / m ka...
Embodiment 3
[0058] Determination of Enzymatic Properties of Recombinant R Conformation Selective Lipase AflB
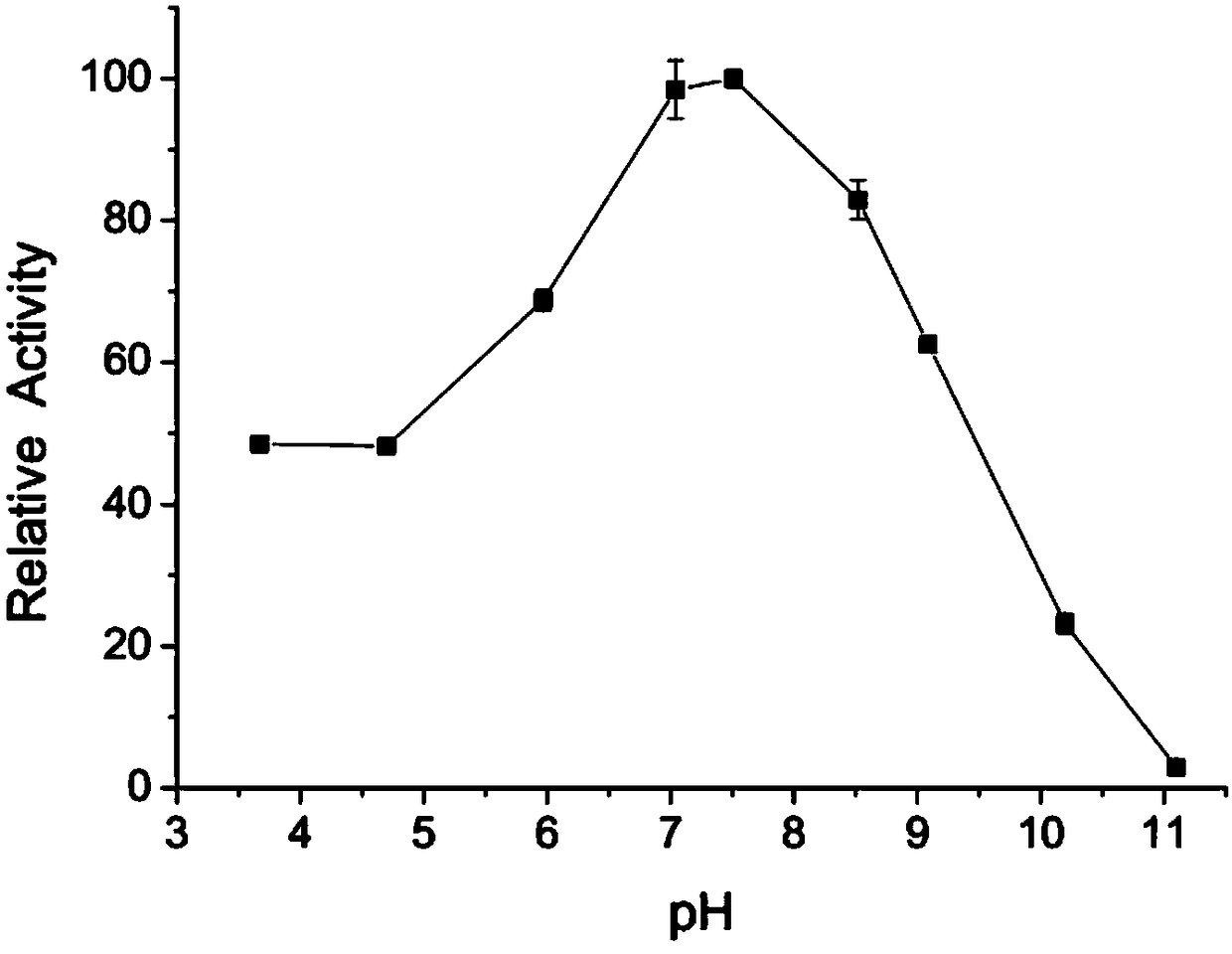
[0059] 1. Optimum reaction pH value of lipase AflB with recombinant R configuration selectivity
[0060] At 40°C, with tributyrin as substrate, pH 3.0, 4.0 and 5.0 (50mM sodium acetate buffer), pH 6.0 and pH 7.0 (50mM phosphate buffer), pH 8.0 (50mM Tris -HCl buffer), pH 9.0 and pH10.0 (50mM glycine buffer) conditions, measure the relative enzyme activity ( figure 2 ).
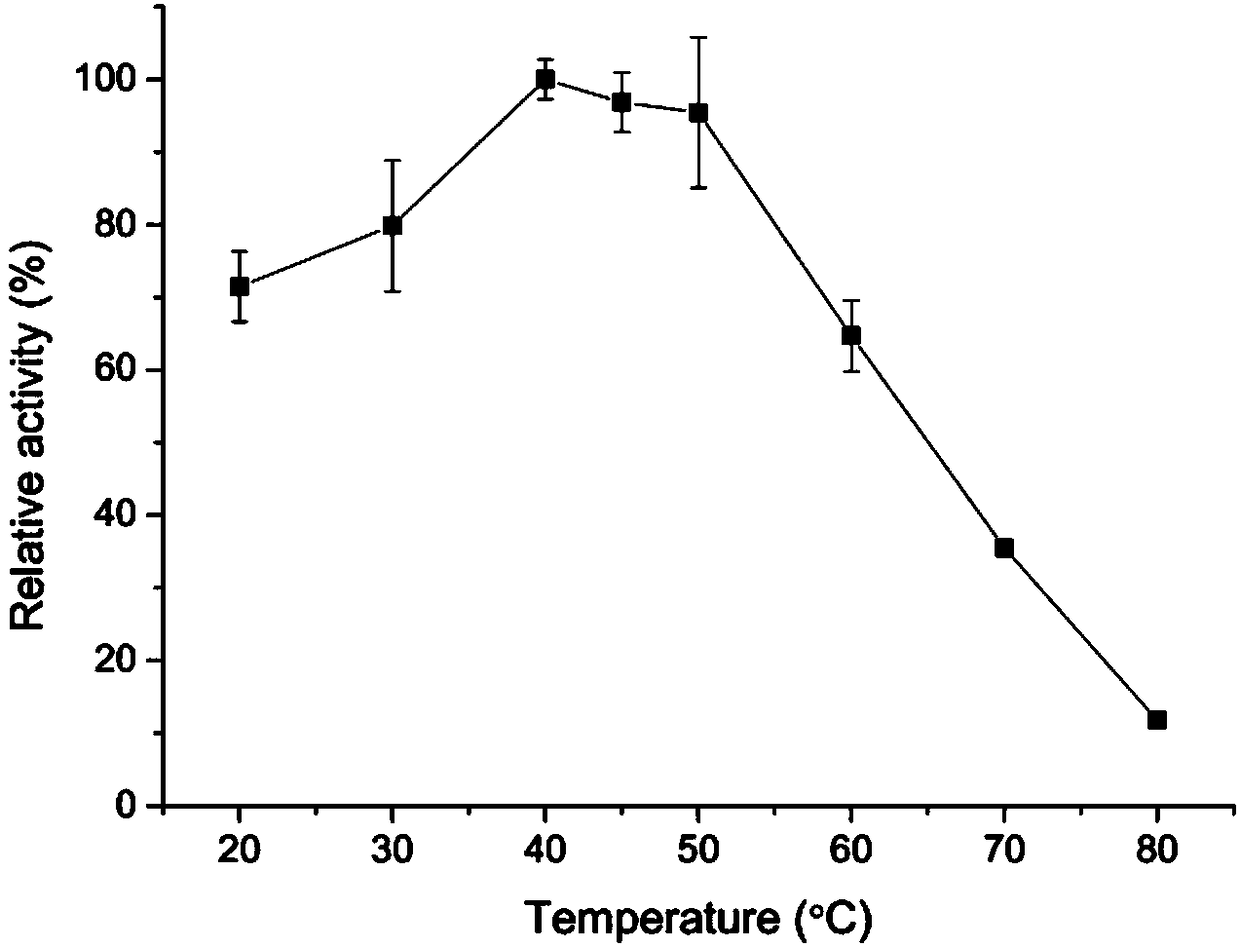
[0061] 2. Optimum reaction temperature of lipase AflB with recombinant R configuration selectivity
[0062] Using tributyrin as a substrate, at pH 7.0 (50mM phosphate buffer), at 20°C, 30°C, 40°C, 45°C, 50°C, 60°C, 70°C and 80°C, respectively Carry out catalytic reaction under the condition, measure the relative enzymatic activity ( image 3 ).
[0063] 3. Selectivity of recombinant R-configuration-selective lipase AflB to substrates with different chain lengths
[0064] Triacetin (TC2), tributyrin (TC4), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com