sj13 polypeptide and its application in the preparation of antithrombotic drugs
A technology for inhibiting coagulation and preparations, applied in the application of antithrombotic drugs, in the field of preparation of anticoagulant, Sj13 polypeptide and its mutants, can solve the problems of weak anticoagulant activity, no anticoagulant polypeptide report, etc., and achieve inhibition of thrombosis. Formation, the development and application value of important anticoagulant/antithrombotic drugs, and the effect of prolonging the detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Construction of Schistosoma japonicum polypeptide vector
[0050] 1. Sj13PCR primer design
[0051] (1) Through the combination of structural biology and bioinformatics, a new Schistosoma japonicum gene / protein was determined from the Schistosoma japonicum gene / protein library, named Sj13, and its polypeptide amino acid sequence is as follows:
[0052] ETLKRYCNLPSDEGICRGYFRRYFYNVTSGECEVFYYGGCLGNRNRFSTIEKCWWYCKGL (SEQ ID NO. 1)
[0053] The protein sequence contains 6 cysteines, which can form 3 pairs of disulfide bonds, which are CysⅠ-CysVI, CysII-CysIV and CysIII-CysⅤ. This pairing mode is a typical feature of protein structure.
[0054] (2) Obtain the cDNA sequence of Sj13 through the sequence reverse translation website, as follows:
[0055] gaaaccctgaaacgctattgcaacctgccgagcgatgaaggcatttgccgcggctattttcgccgctatttttataacgtgaccagcggcgaatgcgaagtgttttattatggcggctgcctgggcaaccgcaaccgctttagcaccatgaaaaatgctggtggtattgcaaaggcctg (SEQ ID NO. 2)
[0056] (3) Use ...
Embodiment 2
[0079] Embodiment 2: Expression and purification of Schistosoma japonicum polypeptide
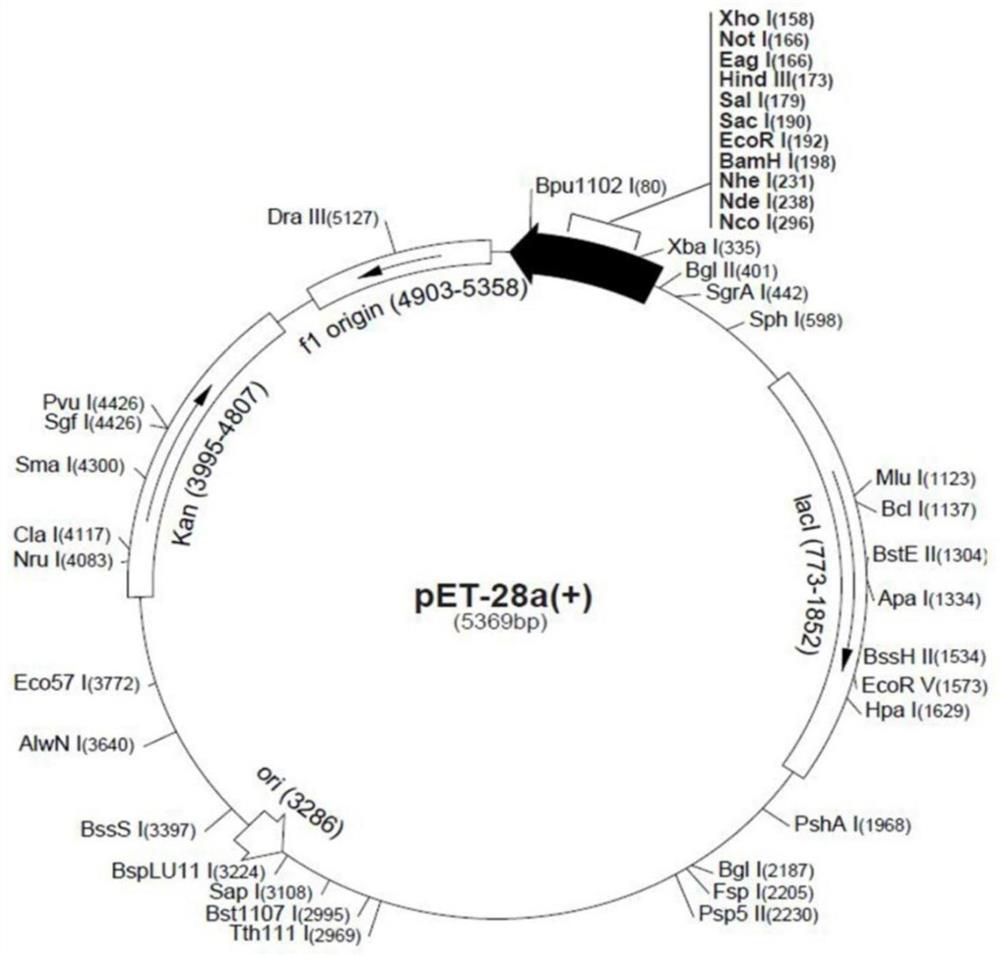
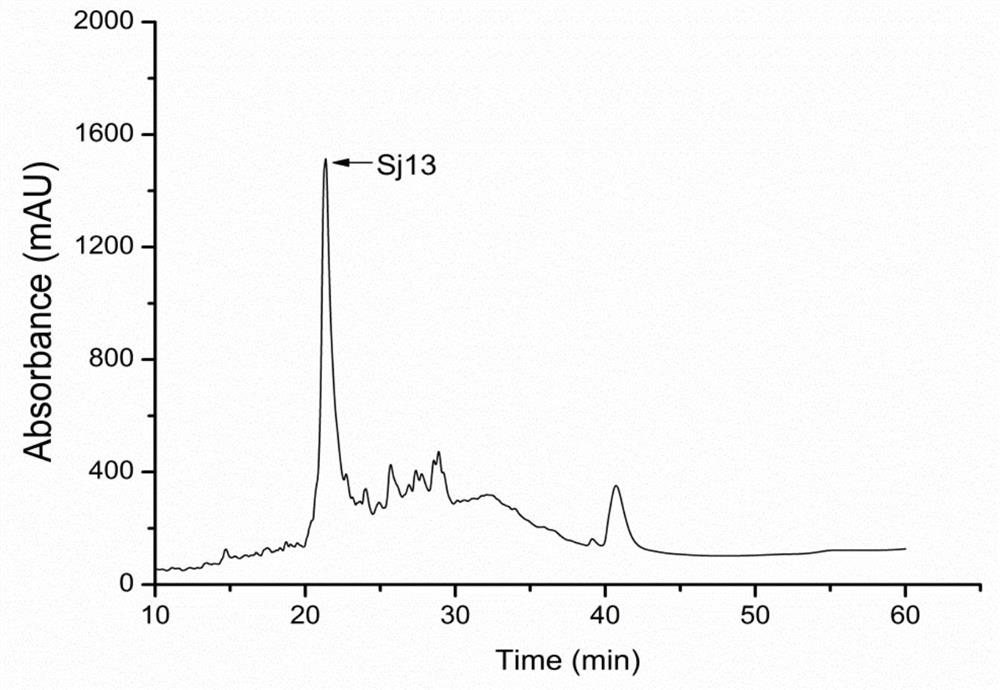
[0080] The constructed pET-28a plasmid was transformed into E.coli Transetta (DE3) expression strains for strain expansion culture to obtain protein inclusion bodies. After the inclusion body was washed, it was denatured, diluted and refolded, concentrated by ultrafiltration and high-performance liquid chromatography (HPLC) to obtain a purified protein solution of the recombinant Schistosoma japonicum protein Sj13. The separation results of high performance liquid chromatography are as follows: figure 2 shown. High performance liquid chromatograph, obvious Sj13 single peak appears, and the protein liquid flowing out under its peak time is all collected, and the purified protein liquid collected is frozen into dry powder with a lyophilizer.
Embodiment 3
[0081] Embodiment 3: BCA quantification of Sj13 protein purification solution
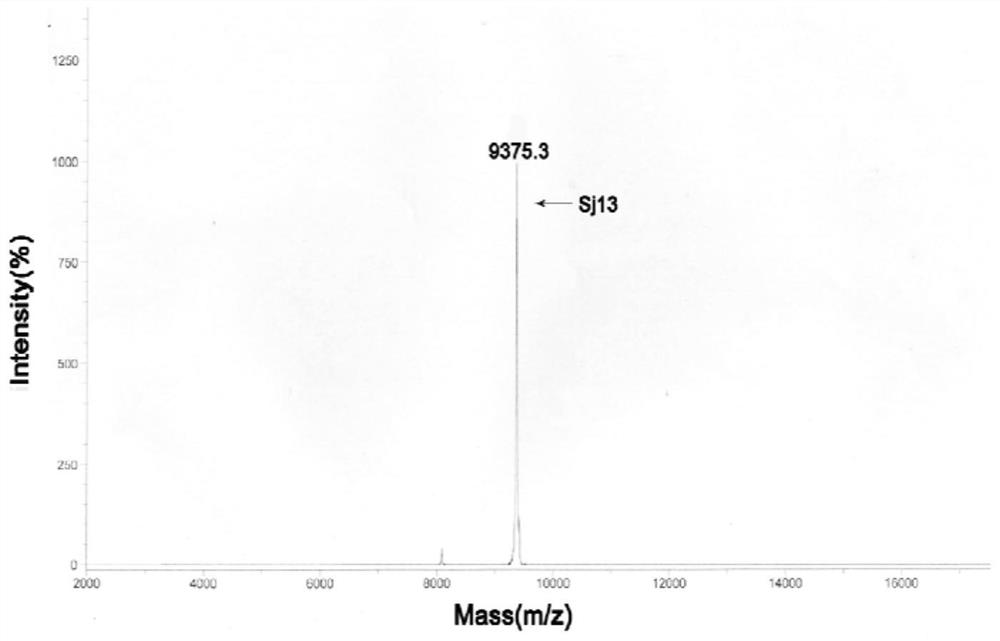
[0082] The BCA protein concentration determination kit (Shanghai Biyuntian Biotechnology Co., Ltd.) was used to quantify the BCA of the purified Sj13 protein solution, and the detected protein concentration was calculated. 10 μg of protein powder was taken out and sent to the Institute of Chemistry, Chinese Academy of Sciences for mass spectrometry detection to further identify whether the protein was recombined successfully. For mass spectrometry results, see image 3 . The mass spectrometry test results provided by the Institute of Chemistry, Chinese Academy of Sciences showed that the molecular weight of the recombinant Sj13 was 9375.3Da; the theoretical molecular weight of Sj13 predicted by the ExPASy-ProtParam tool (http: / / web.expasy.org / protparam / ) website was 9380.52Da. The actual detected value of Sj13 mass spectrometry is consistent with the theoretical value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com