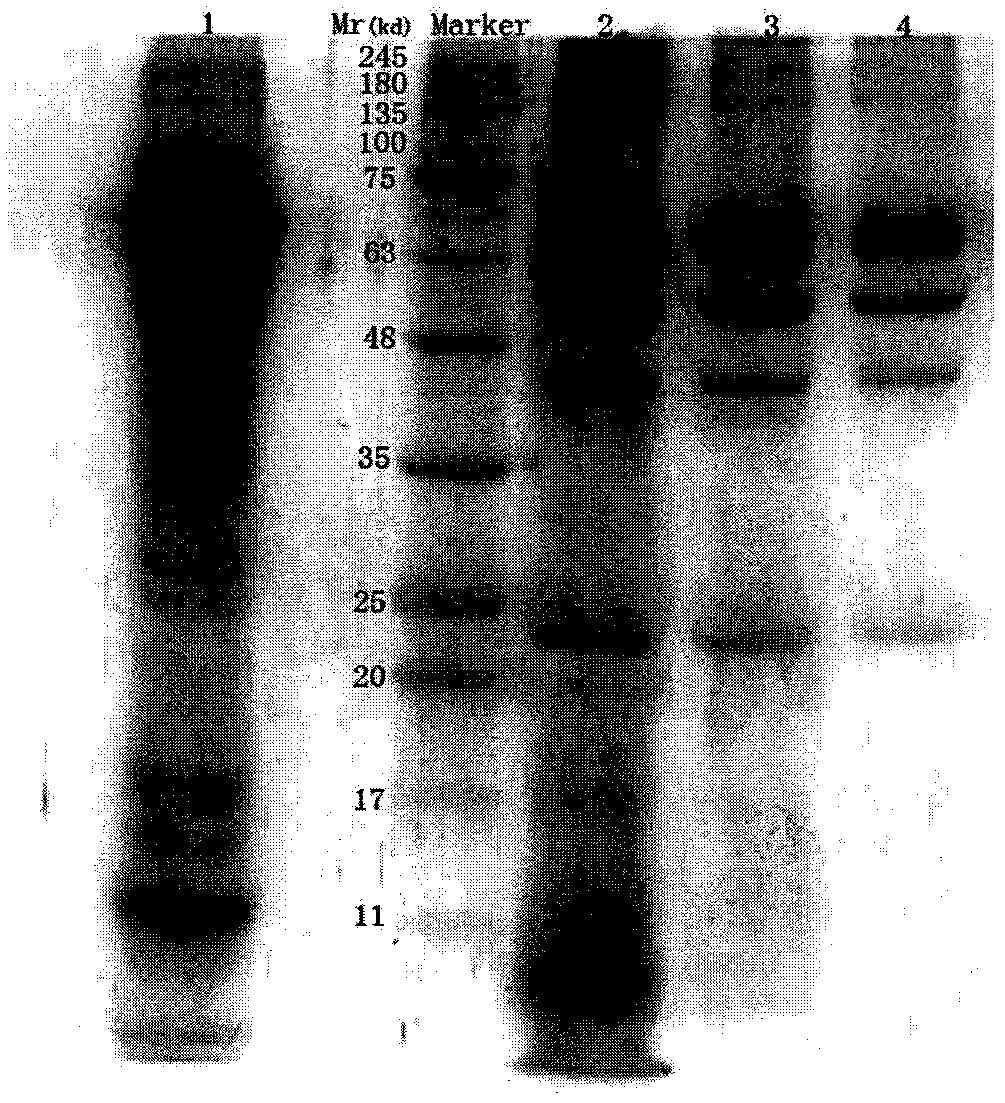Method for producing rabies vaccine
The technology of a rabies vaccine and production method is applied in the field of rabies vaccine production, which can solve the problems of low dosage, high impurity content, and poor correlation of biological potency, etc., and achieve good product stability, simple excipient components, and purification effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Production and product quality detection of embodiment 1 rabies vaccine (Vero cell)
[0103] 1 Single virus harvest solution preparation:
[0104] The bioreactor cultured the Vero cells and the rabies virus CTN-1V strain to prepare the virus harvest liquid (see the above-mentioned virus harvest liquid preparation method 1).
[0105] 2 virus purification
[0106] 2.1 Pretreatment of virus harvest solution
[0107] The single virus harvest was clarified by filtration to remove exfoliated cells and cell debris.
[0108] 2.2 Anion exchange (DEAE column) chromatography
[0109] (1) Column balance: balance the DEAE chromatography column, the balance solution is: 20mmol / L phosphate buffer solution (containing 150mmol / L sodium chloride) of pH 7.6;
[0110] (2) Virus adsorption: add the pretreated virus harvest solution to the equilibrated DEAE chromatography column, and the sample volume is 20 times the column volume; Equilibrium chromatography column;
[0111] (3) Pre-el...
Embodiment 2
[0155] Embodiment 2 Rabies vaccine (human diploid cell) production and product quality detection
[0156] 1 Single virus harvest solution preparation:
[0157] Cultivate MRC-5 cells and rabies virus CTN-1V strain in a square bottle to prepare a virus harvest liquid (see the second preparation method of the aforementioned virus harvest liquid).
[0158] 2 virus purification
[0159] Carry out according to the method described in step 2.1-2.3 of Example 1. According to the enzyme-linked immunosorbent assay results of the virus harvest liquid, increase the amount of DEAE column chromatography in proportion, and reduce the scale of the chromatography column accordingly;
[0160] 3 virus inactivation
[0161] Carry out according to the method described in step 3 of embodiment 1.
[0162] 4 Preparation of vaccine stock solution by desalting
[0163] Carry out according to the method described in step 4 of embodiment 1. Scale down the size of the desalting column according to t...
Embodiment 3
[0180] Embodiment 3 rabies vaccine (chicken embryo) production and product quality detection
[0181] 1 Single virus harvest solution preparation:
[0182] Rabies virus CTN-1V strain was cultured in chicken embryos to prepare virus harvest liquid (see the above-mentioned virus harvest liquid preparation method 3).
[0183] 2 virus purification
[0184] 2.1 Pretreatment of virus harvest solution
[0185]100ml of single virus harvest liquid (chicken embryo allantoic fluid), add 400ml of PBS solution containing 0.1% human serum albumin (sodium phosphate concentration is 20mmol / L, sodium chloride concentration is 150mmol / L, pH value is 7.6), Filter and clarify with a 0.45 μm microporous membrane to remove tissue debris, exfoliated cells and cell debris.
[0186] 2.2 Hydroxyapatite column (CHT column) chromatography
[0187] (1) Column balance: balance the CHT chromatography column, the balance solution is: 20mmol / L phosphate buffer (containing 150mmol / L sodium chloride) with p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com