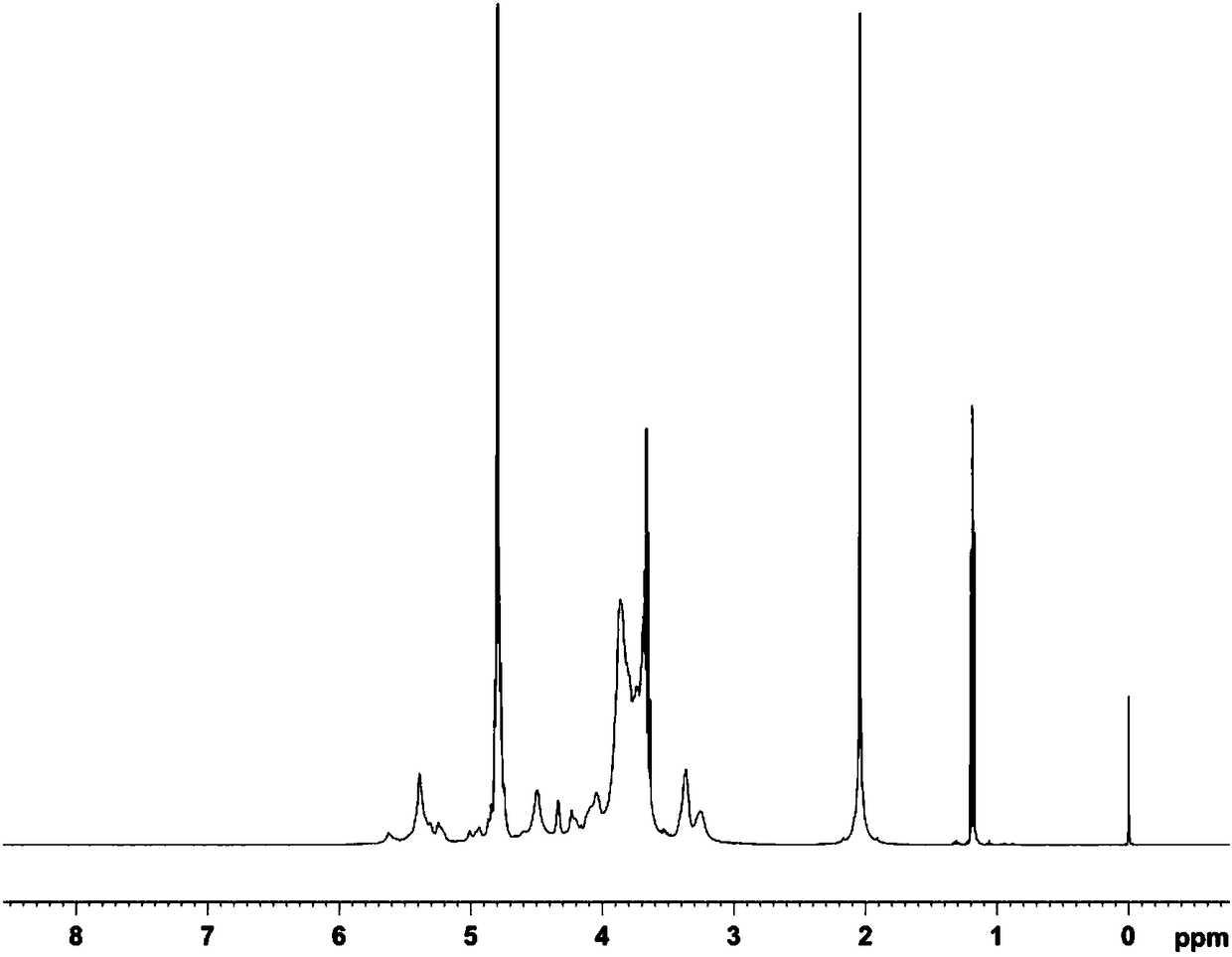
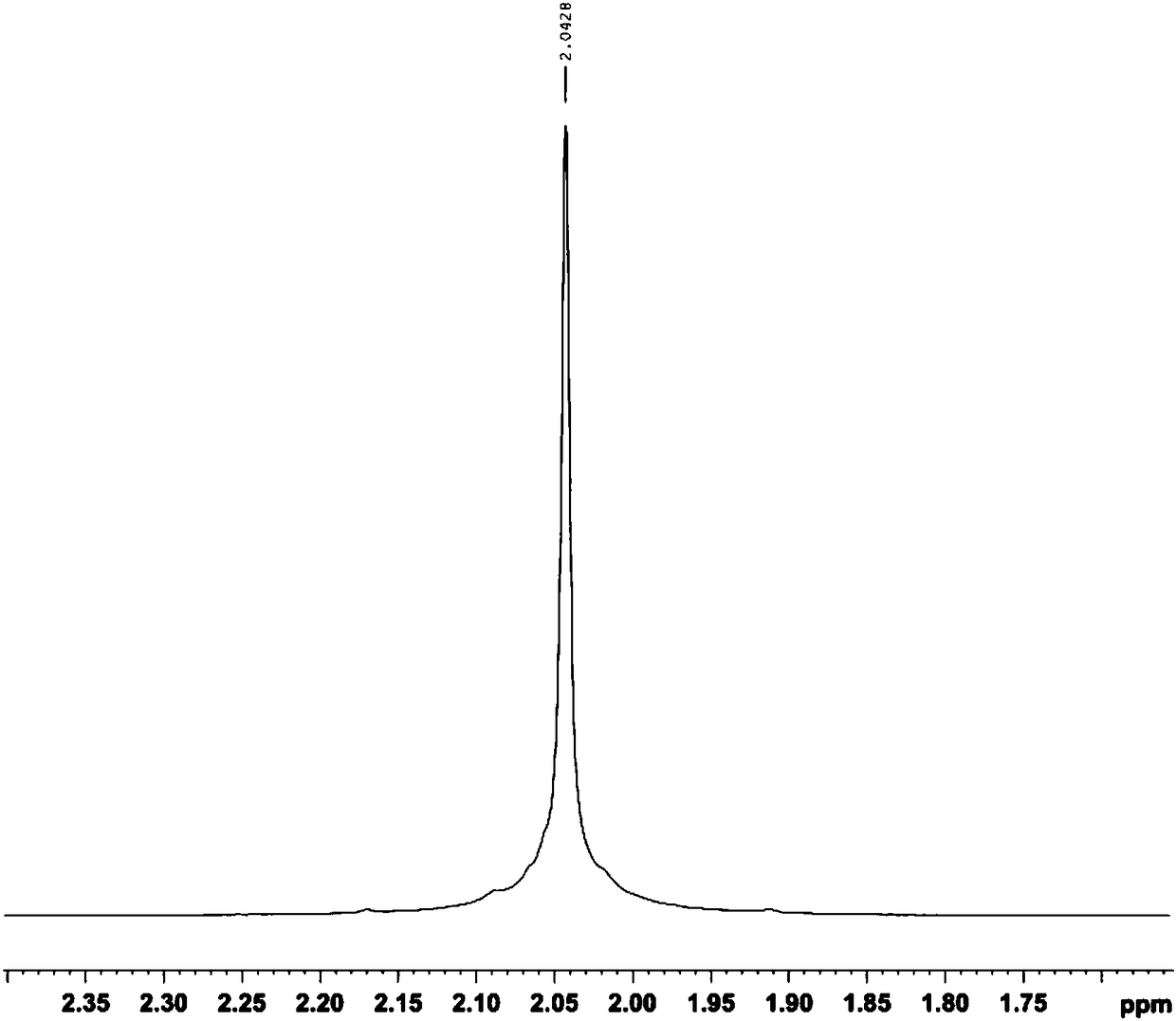
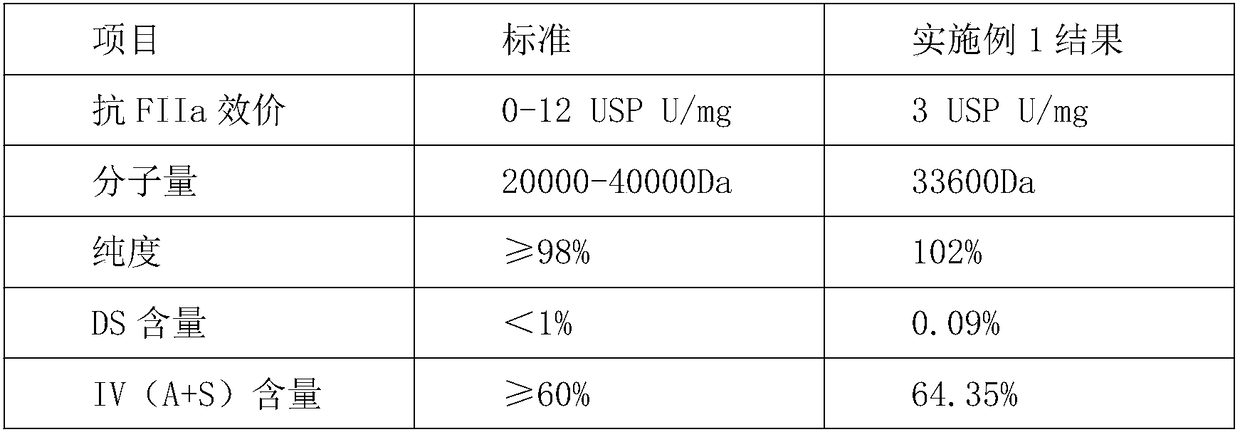
Method for extracting high-purity heparan sulfate from heparan production wastes
A technology of heparan sulfate and waste, which is applied in the extraction of high-purity heparan sulfate and the field of extracting high-purity heparan sulfate. It can solve the problems of low anticoagulant activity, difficult amplification and mixing of process conditions, and solve financial and material resources. , the effect of preserving integrity and creating value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1) Add 1 kg of heparin high-quality production waste, add 19 kg of water, stir and dissolve, and make a solution with a concentration of 5%;
[0046] 2) Acid-adjusting centrifugation: add 1:1 HCl to adjust the pH of the solution to 1.9, add 0.1 times ethanol, stir, and centrifuge;
[0047] 3) Collect the supernatant after centrifugation, add 30% NaOH solution to adjust the pH to 7, and add 2 times more water to make the solution salinity less than 2%;
[0048] 4) Add 50ml of strong basic anion adsorption resin (Rigson resin, USA) to the above solution, stir and adsorb for 18h;
[0049] 5) Separating the remaining adsorption liquid and the resin, washing the collected resin with water, then adding 0.5 times the volume of 3% sodium chloride solution for elution, collecting the eluate after 8 hours of elution, adding 0.8 times the volume of ethanol for precipitation;
[0050] 6) Collect the precipitate, add 3 times of water to dissolve, add hydrogen peroxide to oxidize, ...
Embodiment 2
[0057] 1) Add 1 kg of heparin high-quality production waste, add 5.67 kg of water, stir and dissolve, and make a solution with a concentration of 15%;
[0058] 2) Acid-adjusting centrifugation: add sulfuric acid solution to adjust the pH of the solution to 2.0, add 0.7 times of ethanol, stir, and centrifuge;
[0059] 3) Collect the centrifuged supernatant, add 30% NaOH solution to adjust the pH to 6.0, and add 2.5 times more water to make the solution salinity less than 2%;
[0060] 4) Add 500ml of strongly basic anion adsorption resin (Germany LANXESS resin) to the above solution, stir and adsorb for 13h;
[0061] 5) Separating the remaining adsorption liquid and the resin, washing the collected resin with water, then adding 2 times the volume of 7% sodium chloride solution for elution, collecting the eluate after 2 hours of elution, adding 0.6 times the weight acetone for precipitation;
[0062] 6) Collect the precipitate, add 3 times of water to dissolve, add hydrogen per...
Embodiment 3
[0069] 1) Add 1 kg of heparin high-quality production waste, add 9 kg of water, stir and dissolve, and make a solution with a concentration of 10%;
[0070] 2) Acid-adjusting centrifugation: add trichloroacetic acid solution to adjust the pH of the solution to 2.3, add 0.35 times of ethanol, stir, and let stand for 13 hours;
[0071] 3) Collect the supernatant after standing still, add 30% NaOH solution to adjust the pH to 8, and add 2 times more water to make the solution salinity less than 2%;
[0072] 4) above-mentioned solution loads on the ion-exchange chromatography column that 500ml strong anion-exchange gel Q Sepharose is housed;
[0073] 5) Add 0.5 times the volume of 0.6M sodium chloride solution for elution, the gravity flow rate, collect the eluate, add 1 times the weight of methanol for precipitation;
[0074] 6) Collect the precipitate, add 3 times of water to dissolve, add hydrogen peroxide to oxidize, and oxidize for 48 hours.
[0075] 7) After oxidation, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| gel fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com