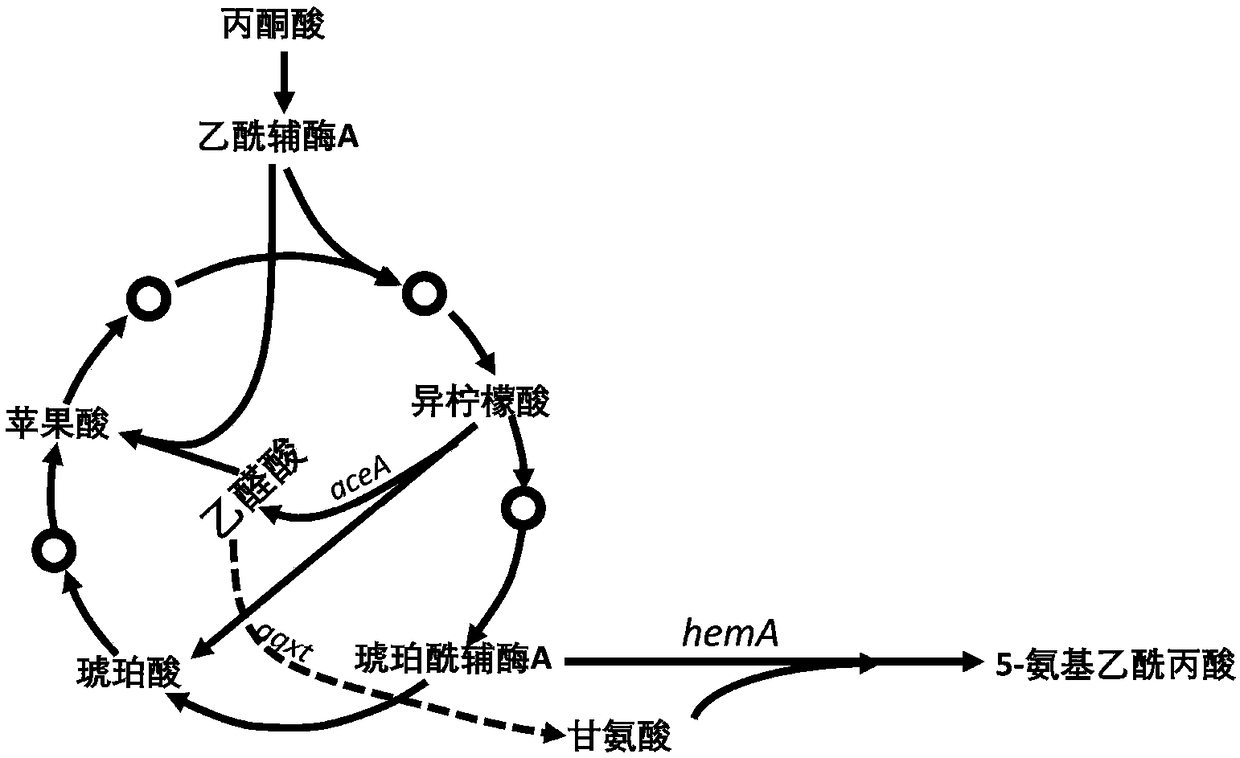
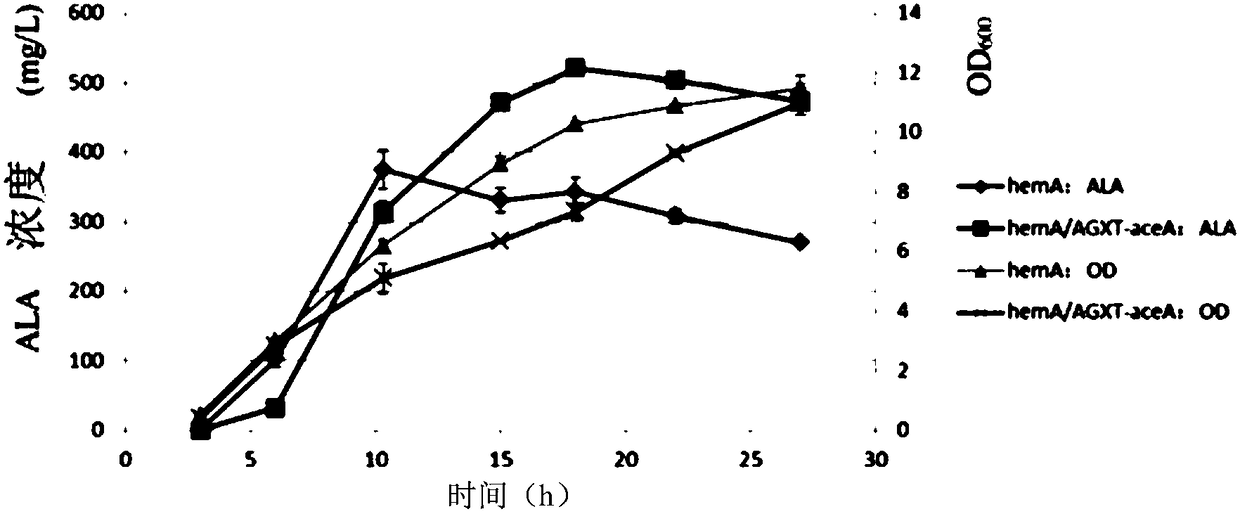
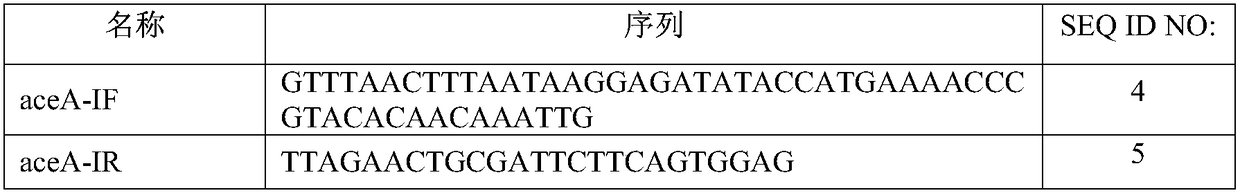
Glyoxylate aminotransferase mediated biosynthesis pathway of 5-aminolevulinic acid
A technology of glyoxylate transaminase and glycine transaminase, which is applied in the field of genetic engineering and microbial fermentation, can solve the problems of loss of carbon source, long synthesis pathway, high toxicity of reaction solvent, etc., and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: In vitro verification of new pathways for 5-ALA biosynthesis
[0039] ①The glyoxylate transaminases from different sources were connected to the expression vector pet21a to construct recombinant expression vectors, respectively: alanine-glyoxylate transaminase pet21a-AGXT derived from Homo sapiens; Saccharomyces cerevisiae Alanine-glyoxylate transaminase pet21a-ScTA derived from Hyphomicrobium methylovorum; Alanine-glyoxylate transaminase pet21a-HmTA derived from Hyphomicrobium methylovorum;
[0040] ② Construction of isocitrate lyase gene expression vector pet21a-aceA;
[0041] ③ Construct the expression vector pet22b-sucCD of succinyl-CoA synthetase;
[0042] ④ Construct the expression vector pet22b-hemA of 5-ALA synthetase.
[0043] Transform the above expression vectors into the E. coli expression host E.coli BL21star (DE3), ferment at 37°C until the OD reaches 0.5-0.6, add 0.1mM IPTG to induce gene expression, continue to culture at 30°C for 10 hours, an...
Embodiment 2
[0046] Example 2: Construction of plasmids containing 5-ALA synthetase gene, glyoxylate transaminase gene and isocitrate lyase gene
[0047] (1) Gene synthesis and amplification: the 5-ALA synthetase gene hemA (shown in SEQ ID NO.1) and the glyoxylate transaminase gene AGXT (shown in SEQ ID NO.2) derived from Rhodobacter sphaeroides were combined (shown) were sent to Nanjing GenScript Biotech Co., Ltd. for synthesis to obtain plasmids pUC-hemA and pUC-AGXT. The isocitrate lyase gene aceA was amplified from Escherichia coli MG1655 with primers aceA-IF and aceA-IR for future use.
[0048] (2) Construction of plasmid pETDuet-1-hemA: the vector fragment (backbone) was amplified from plasmid pETDuet-1 with primers ACYCDuet-VecF and DuetDOWN1, and the insert fragment was amplified from plasmid pUC-hemA with primers DuetUP2 and T7Terminator (In-hemA), after the two fragments were purified and recovered, they were connected by means of Gibson assembly (Gibson assembly). The ligation...
Embodiment 3
[0053] Embodiment 3: Containing the construction of the recombinant strain of 5-ALA biosynthesis new pathway
[0054] Insert 100 μL of E. coli BL21star (DE3) into 20 mL of LB medium and store 100 μL of glycerol strains. Cultivate at 37 ° C and 220 r / min for 5 hours until the OD value reaches 0.5. Centrifuge 2 mL of the bacterial liquid, discard the supernatant, and The cell pellet was washed by re-spinning with 0.1M calcium chloride, the supernatant was discarded after centrifugation, and the washing was repeated. The cell pellet was re-spinned with 0.1M calcium chloride to obtain E.coliBL21star (DE3) cell competent cells.
[0055] Plasmid pETDuet-1-hemA and plasmid pRSFduet-1-AGXT-aceA were extracted from ToP10 (pETDuet-1-hemA) and ToP10 (pRSFduet-AGXT-aceA) obtained in Example 2, respectively.
[0056] Add 2 μL pETDuet-1-hemA plasmid to E.coli BL21star (DE3) competent cells, let it stand on ice for 30 minutes, place it in a water bath at 42°C for 90 seconds, then place it on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com