Method for extracting ergosterol from starch yeast
An ergosterol and starch fermentation technology, applied in the directions of steroids, organic chemistry, etc., can solve the problems of difficult three-waste treatment, insufficient market supply, cumbersome process steps, etc., and achieves less harmful impurities, less waste of resources, and short technological process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
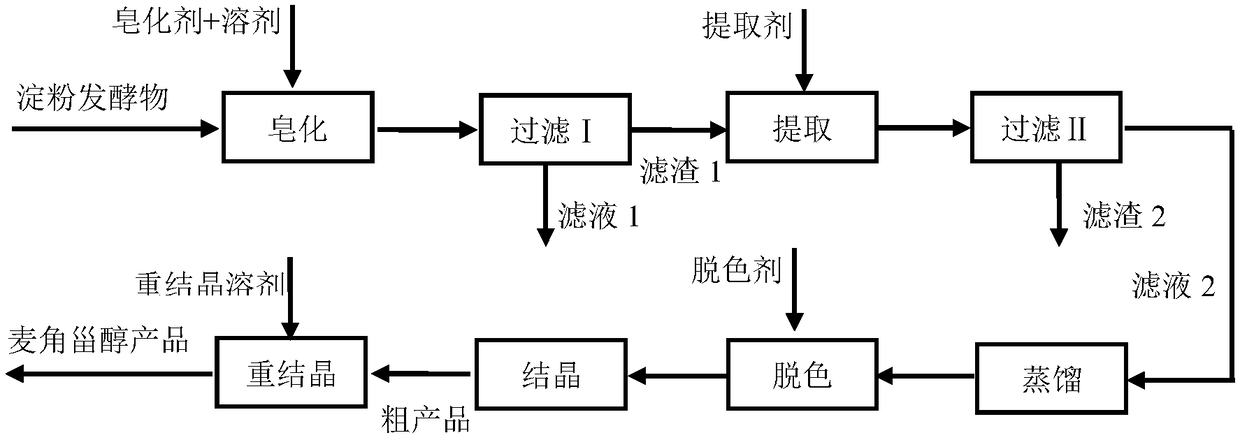
[0048] (1) Saponification: Weigh 1kg of the fermented starch product, add it to a stirred container, add 1.2kg of ethanol, heat to maintain the temperature at 50-60°C, add 70g of potassium hydroxide, and stir for 16 hours. Then cool to room temperature.
[0049] (2) Filtration I: Ethanol is recovered by vacuum filtration.
[0050] (3) Extraction: Add the filter residue in step (2) into a stirred container, add 1.2 kg of ethyl acetate, heat to maintain the temperature at 50-60° C., and stir for 8 hours. Then cool to room temperature.
[0051] (4) Filtration II: vacuum filtration is used to collect the filtrate.
[0052] (5) Distillation: the filtrate collected in the step (4) is heated and distilled under normal pressure, and ethyl acetate is recovered by condensation. The residue was cooled to room temperature and filtered to obtain a light brown solid.
[0053] (6) Decolorization: Add the crystals obtained in step (5) into an airtight container, add 750 g of chloroform, s...
Embodiment 2
[0056] (1) Saponification: Weigh 5 kg of starch fermented product, add it to a stirring container, add 1.8 kg of methanol and 4.2 kg of methylene chloride; heat to maintain the temperature at 35-45°C, add 250 g of sodium hydroxide, and stir for 12 hours. Then cool to 25°C.
[0057] (2) Filtration I: Recover methanol and dichloromethane by vacuum filtration.
[0058] (3) Extraction: Add the filter residue in step (2) into a stirred container, add 5 kg of chloroform, heat to maintain the temperature at 50-60° C., and stir for 8 hours. Then cool to room temperature.
[0059] (4) Filtration II: vacuum filtration is used to collect the filtrate.
[0060] (5) Distillation: the filtrate collected in the step (4) is heated and distilled under normal pressure, and condensed to recover chloroform. The residue was cooled to room temperature and then filtered to obtain 4.7 g of crude ergosterol.
Embodiment 3
[0062] (1) Saponification: Weigh 1 kg of starch fermented product, put it into a stirred container, add 1.2 kg of methanol, heat to maintain the temperature at 50-55 ° C, add 50 g of sodium hydroxide, and stir for 18 hours. Then cool to room temperature.
[0063] (2) Filtration I: adopt vacuum filtration to recover methanol.
[0064] (3) Extraction: Add the filter residue in step (2) into a stirred container, add 1.2 kg of petroleum ether, heat to maintain the temperature at 40-50° C., and stir for 18 hours. Then cool to room temperature.
[0065] (4) Filtration II: vacuum filtration is used to collect the filtrate.
[0066] (5) Distillation: the filtrate collected in step (4) is heated and distilled under normal pressure, and petroleum ether is recovered by condensation. The residue was cooled to room temperature and then filtered to obtain a light brown ergot crude product.
[0067] (6) Decolorization: Add the crude product obtained in step (5) into an airtight container...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com