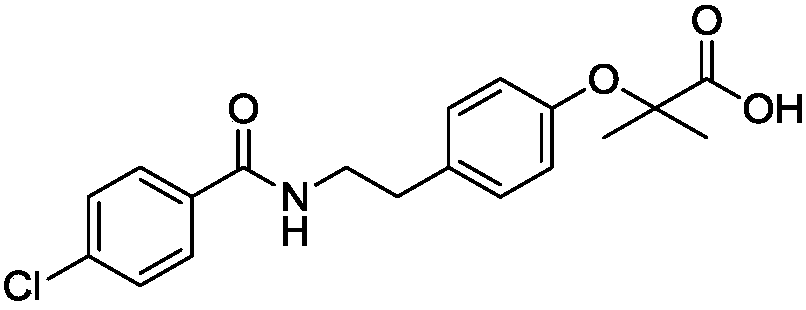
Preparation process of bezafibrate compound
A preparation process and technology of bezafibrate, applied in the field of pharmaceutical synthesis and preparation of bezafibrate, can solve the problems of easy hygroscopicity of sodium hydroxide, many side reactions, affecting the progress of the reaction, etc., so as to avoid a large loss of products, overcome the Explosion hazard and the effect of improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
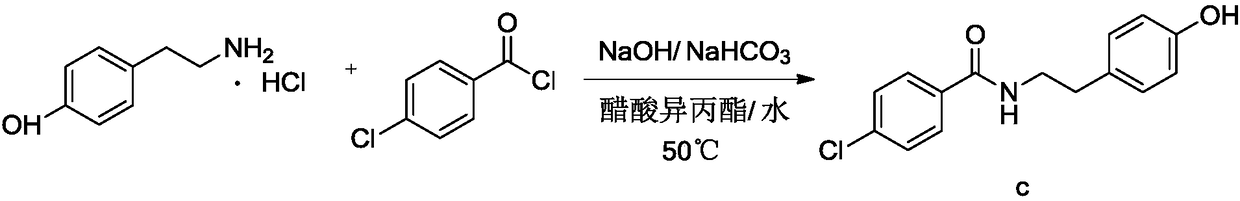
[0060] Embodiment 1: the preparation of N-p-hydroxyphenethyl-4-chlorobenzamide
[0061]
[0062] Add 250g of water and 40.0g of p-hydroxyphenylethylamine hydrochloride into a 500ml reaction bottle, add 30.7g of 30% sodium hydroxide under stirring, add 26.2g of sodium bicarbonate, cool down to 10-15°C, and dropwise add 42.0g of p-chloro Benzoyl chloride was dropped in about 3 hours. After the reaction was completed, it was filtered and dried to obtain the crude product of N-p-hydroxyphenethyl-4-chlorobenzamide with a yield of 97.6%.
Embodiment 2
[0063] Embodiment 2: the preparation of N-p-hydroxyphenethyl-4-chlorobenzamide
[0064]
[0065] Add 250g of water and 30.0g of p-hydroxyphenylethylamine into a 500ml reaction bottle, add with stirring, add 18.4g of sodium bicarbonate, cool down to 10-15°C, add 42.0g of p-chlorobenzoyl chloride dropwise, and drop it in about 3 hours , the reaction was completed, filtered and dried to obtain the crude product of N-p-hydroxyphenethyl-4-chlorobenzamide with a yield of 97.8%.
Embodiment 3
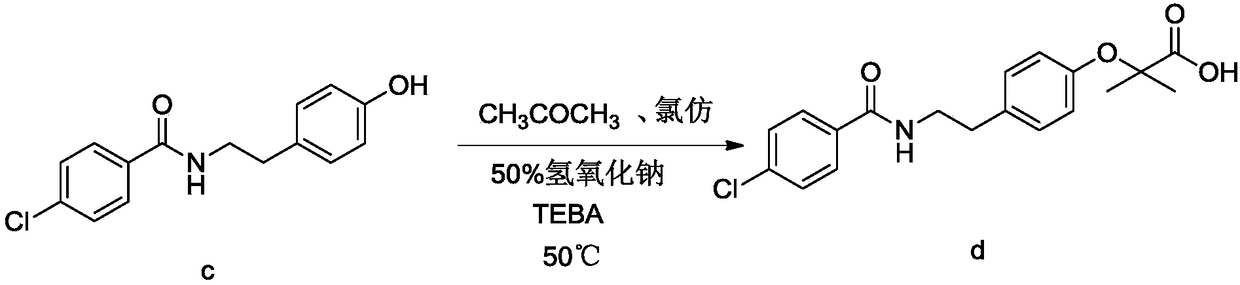
[0066] Embodiment 3: the preparation of bezafibrate
[0067]
[0068] Add 820g of acetone and 50.0g of acylate into a 2000ml reaction flask, raise the temperature to 50-60°C, stir to dissolve, add 129.6g of sodium hydroxide solution, add 64.9g of chloroform dropwise at 50-60°C, drop for about 1 hour After completion, keep warm for half an hour, distill at normal pressure, recover acetone, add 600g of water after distillation, dissolve, cool down to 0-5°C to crystallize, filter to get sodium salt wet product, add water to dissolve the wet product, add hydrochloric acid solution to adjust to acidity, Filter and dry to obtain the finished product of bezafibrate with a yield of 85%, a purity of 99.5%, and mp: 181-182°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com