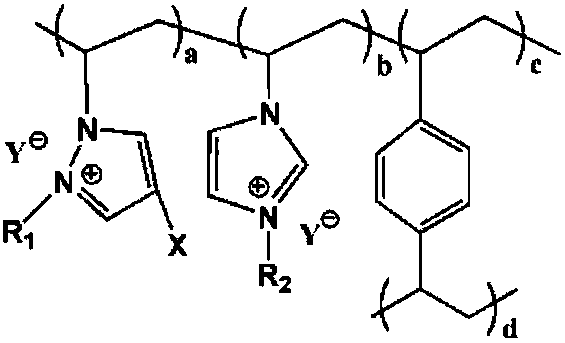
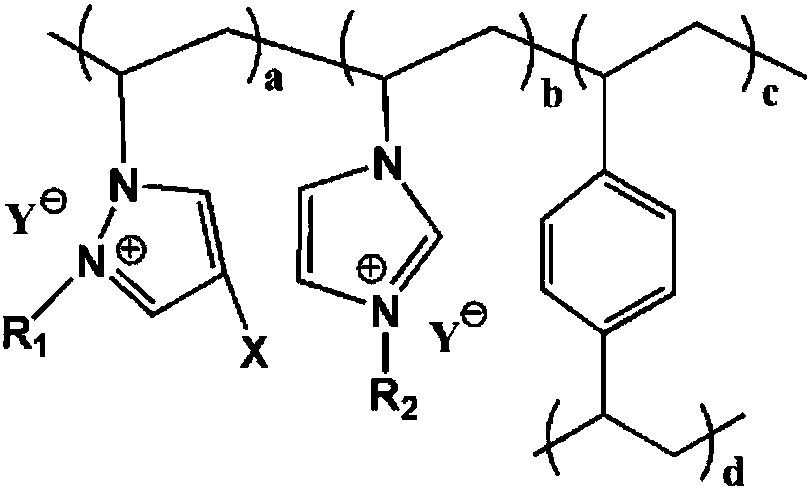
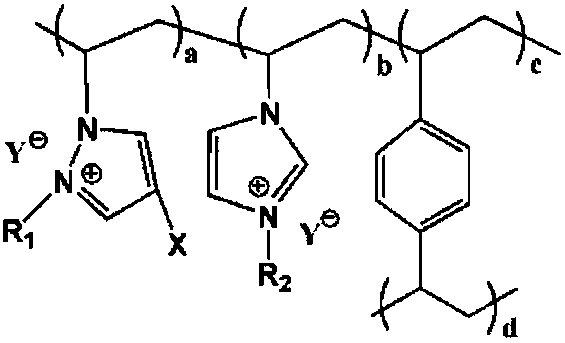
Cross-linking polymerization acidic ionic liquid alkylating catalyst and preparation method thereof
An acidic ionic liquid and cross-linking polymerization technology, which is applied in the direction of carbon compound catalysts, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the problem that active components are easy to fall off and catalysts are easy to deactivate and other problems, to achieve clear network structure, improve catalytic reaction efficiency, and achieve the effect of large pore volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0044] 25°C, N 2 Under atmosphere, 1.73g 4-halo-1-vinylpyrazole, 2.05g 1-vinyl-3-ethylimidazolium bromide, 2.60g crosslinking agent divinylbenzene, 0.19g azobisisobutyl Nitrile and 22.55 g of ethyl acetate were added to the reaction kettle and mixed evenly, and the temperature was raised to 80°C for 36 hours, and the ethyl acetate was distilled off under reduced pressure at 60°C, vacuum-dried at 40°C for 24 hours, and cooled to room temperature to obtain a cross-linked polymeric ionic liquid , put 2.63g of n-bromobutane and 25.95g of absolute ethanol into the reaction kettle, react at room temperature for 24h, filter, wash the filter cake with 23.70g of absolute ethanol each time, wash 3 times in total, and vacuum dry at 50°C for 8h to obtain Substitute cross-linked polymeric ionic liquid; at room temperature, add the substituted cross-linked polymeric ionic liquid, 18.34g 2mol / L toluene trifluoroacetic acid solution into the reaction kettle and soak for 24h, filter, and filte...
Embodiment example 2
[0047] 26°C, N 2 Under the atmosphere, 2.20g 4-iodo-1-vinylpyrazole, 3.74g 1-vinyl-3-butyl imidazolium bromide, 4.61g crosslinking agent divinylbenzene, 0.53g azobisisobutyl Nitrile and 26.77g of acetone were added to the reaction kettle and mixed evenly, and the temperature was raised to 80-120°C for 12-36 hours, the acetone was distilled off under reduced pressure at 60-100°C, vacuum-dried at 60°C for 12 hours, and cooled to room temperature to obtain crosslinking To polymerize ionic liquid, add 4.22g n-bromobutane and 30.91g absolute ethanol into the reaction kettle, react at room temperature for 12h, filter, wash the filter cake with 27.88g absolute ethanol each time, wash 4 times in total, and vacuum dry at 60°C 6h, to obtain a substituted cross-linked polymeric ionic liquid; at room temperature, add the substituted cross-linked polymeric ionic liquid, 25.11g 5mol / L toluene trifluoromethanesulfonate solution into the reactor and soak for 24h, filter, and use the filter ca...
Embodiment example 3
[0050] 20°C, N 2 Under the atmosphere, 2.60g 4-bromo-1-vinylpyrazole, 3.22g 1-vinyl-3-hexylimidazolium bromide, 5.63g crosslinking agent divinylbenzene, 0.92g dibenzoyl peroxide Add 32.77g of isopropanol into the reaction kettle and mix well, heat up to 100°C and react for 24h, distill under reduced pressure at 80°C to remove isopropanol, vacuum dry at 50°C for 20h, and cool down to room temperature to obtain a cross-linked polymeric ionic liquid , add 4.72g of n-bromohexane and 33.28g of absolute ethanol into the reaction kettle, react at room temperature for 20h, filter, wash the filter cake with 30.42g of absolute ethanol each time, wash 3 times in total, and vacuum dry at 80°C for 7h to obtain Substitute cross-linked polymeric ionic liquid; at room temperature, add the substituted cross-linked polymeric ionic liquid, 42.33g 1mol / L toluene trifluoroacetic acid solution into the reaction kettle for immersion for 36h, filter, and filter the cake with 35.49g anhydrous diethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com