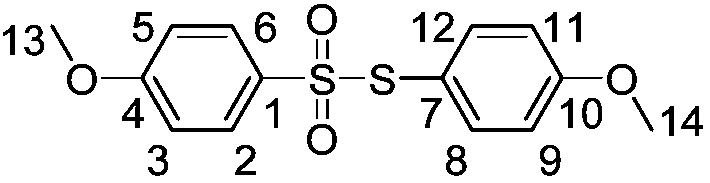Synthetic method for preparing thiosulfonates on basis of sulfinic acid sodium salt disproportionated reaction
A technology of thiosulfonate and sodium sulfinate, which is applied in the preparation of sulfonic acid amide, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of complex synthesis conditions, etc. Compositing fast effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
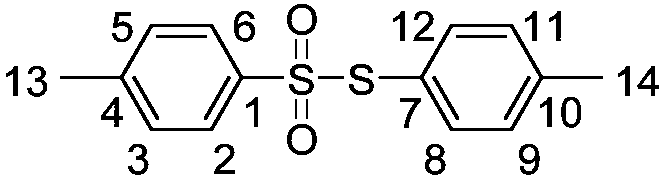
[0022] With 0.356 grams of sodium p-toluene sulfinate as raw material, the amount of accelerator boron trifluoride diethyl ether is 0.852 grams, and 8 milliliters of dichloromethane as solvent, at a temperature of 50 ° C, react together for 3 hours, through Diluted with water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain 0.245 grams of white solid thiosulfonate product with a yield of 88%. Confirmed by organic matter characterization methods such as spectroscopy and mass spectrometry.
[0023] The structure of product is as follows (the letter mark on the structural formula is corresponding to the label in the nuclear magnetic test data):
[0024]
[0025] Compound characterization data: white solid, m.p.73.8-75.2℃; 1 H NMR (400MHz, CDCl 3 ),δ:2.37(s,3H,ArCH 3 -14),2.42(s,3H,ArCH 3 -13),7.14(d,J=8.0Hz,2H,ArH-9,11),7.21(d,J=8.0Hz,2H,ArH-8,12)7.24(d,J=8.0Hz,2H, ArH-3,5), 7.45 (d, J=8.0Hz, 2H, ArH-2,...
Embodiment 2
[0027] With 0.388 grams of sodium p-methoxybenzene sulfinate as raw material, the amount of accelerator boron trifluoride diethyl ether is 0.852 grams, and 8 milliliters of dichloromethane as solvent, at a temperature of 50 ° C, react together for 3 hours, Diluted with water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain 0.251 g of white solid thiosulfonate product with a yield of 81%. Confirmed by organic matter characterization methods such as carbon spectrum and mass spectrometry.
[0028] The structure of product is as follows (the letter mark on the structural formula is corresponding to the label in the nuclear magnetic test data):
[0029]
[0030] Compound characterization data: white solid, m.p.73.8-75.2℃; 1 HNMR (400MHz, CDCl 3 ),δ:3.82(s,3H,ArCH 3 -14),3.86(s,3H,ArCH 3-13),6.84(d,J=8.0Hz,2H,ArH-3,5),6.87(d,J=8.0Hz,2H,ArH-9,11),7.27(d,J=8.0Hz,2H ,ArH-8,12),7.50(d,J=8.0Hz,2H,ArH-2,6); 13 ...
Embodiment 3
[0032] With 0.441 grams of sodium 2,3,5,6-tetramethylbenzenesulfinate as raw material, the amount of accelerator boron trifluoride diethyl ether is 0.852 grams, 8 milliliters of dichloromethane as solvent, at a temperature of 50 ° C , reacted together for 3 hours, diluted with water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain 0.276 g of white solid thiosulfonate product with a yield of 76%. Confirmed by the characterization methods of organic matter such as hydrogen nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum, mass spectrometry, and elemental analysis.
[0033] The structure of product is as follows (the letter mark on the structural formula is corresponding to the label in the nuclear magnetic test data):
[0034]
[0035] Compound characterization data: white solid, m.p.135.3-136.8°C; 1 H NMR (400MHz, CDCl 3 ),δ:2.13(s,6H,ArCH 3 -17,20),2.16(s,6H,ArCH 3 -13,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com