Dianhydride monomer with side chains containing phenylacetylene and synthesis method and application of dianhydride monomer
A technology of acetylene dianhydride and ethynyl biphenyl dianhydride, which is applied in the field of polyimide synthesis and can solve the problems of low BET specific surface area, limited carbon dioxide adsorption performance, and further improvement of cross-linking degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
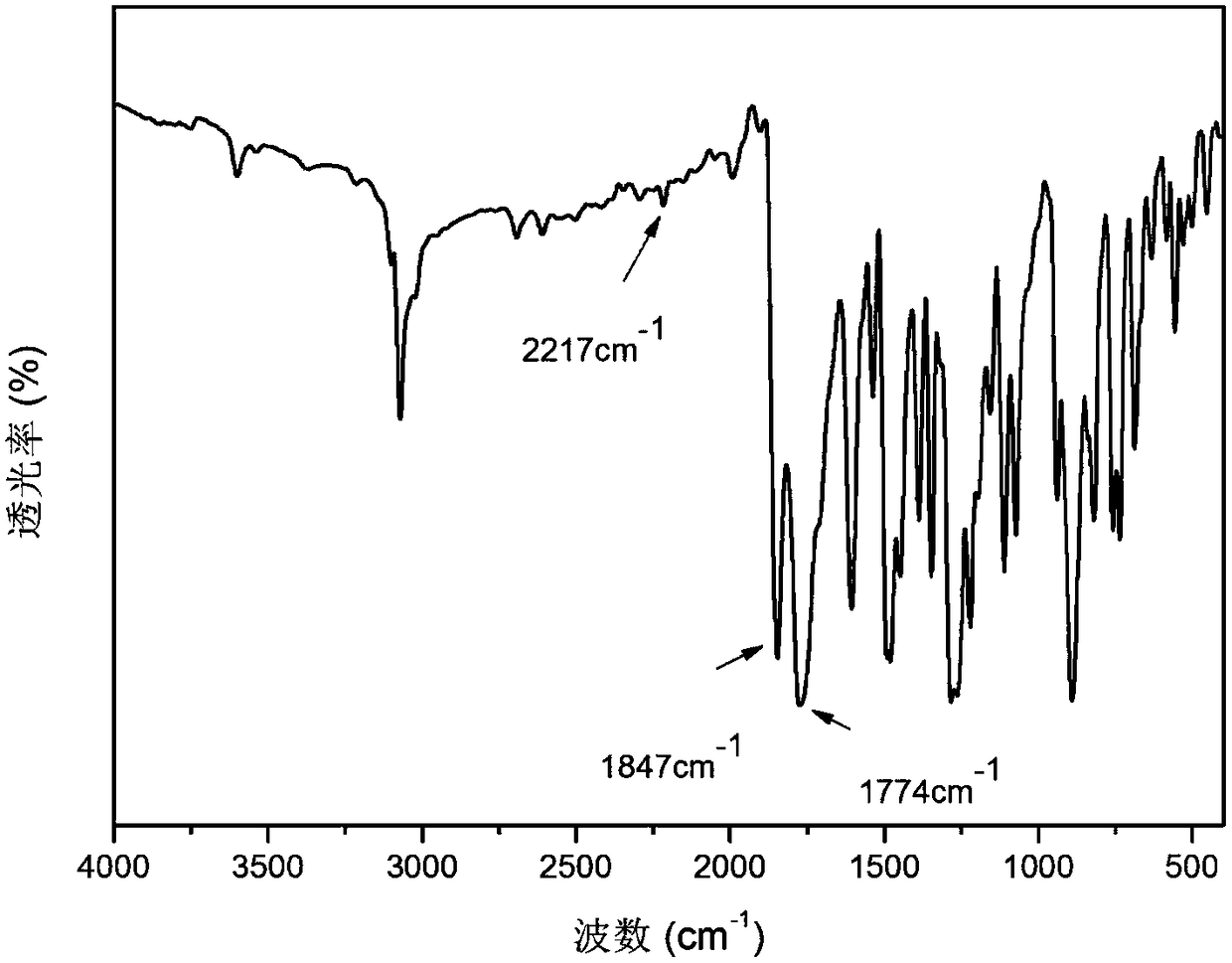
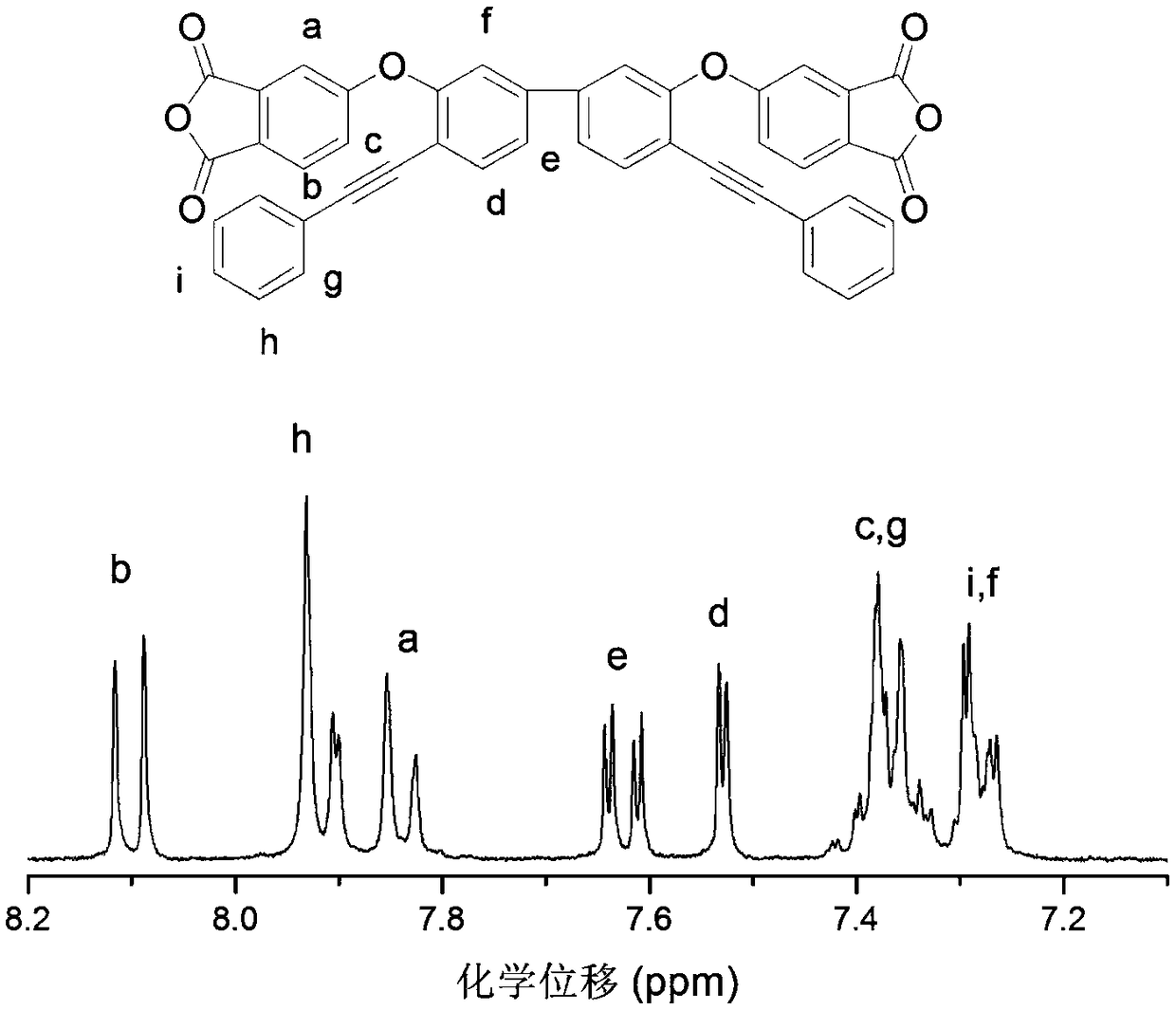
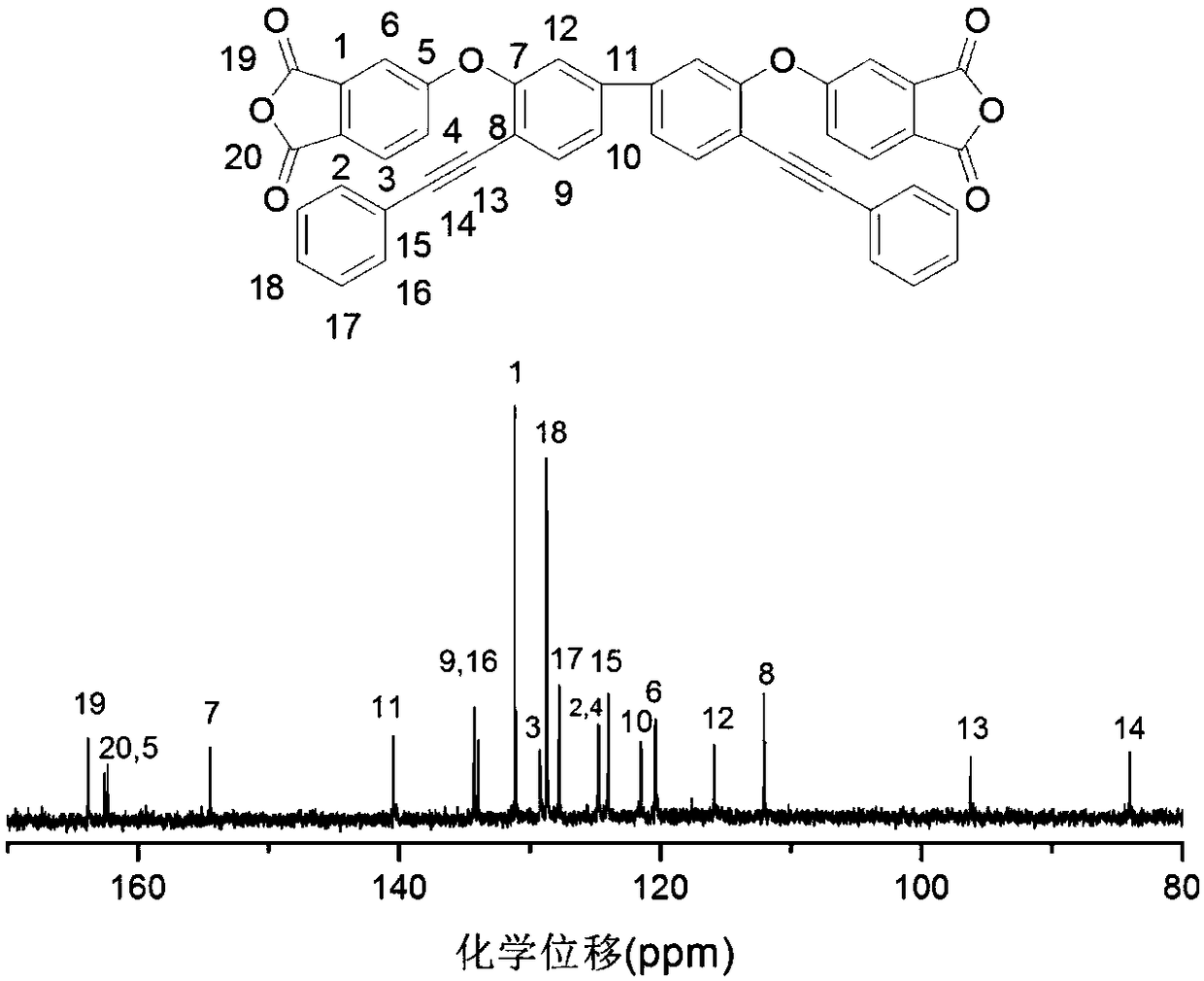
[0036] This example illustrates the synthesis and preparation steps of 3,3'-bis(3,4-dicarboxyphenoxy)-4,4'-tolanyl biphenyl dianhydride, specifically as follows:
[0037] Step 1: Add 3,3'-dihydroxybenzidine (A) and hydrogen bromide solvent into a 500mL three-necked flask equipped with a nitrogen port and a constant pressure dropping funnel, and dissolve sodium nitrite in deionized water. Slowly add sodium nitrite solution dropwise into a three-necked flask at -5 ~ 0°C to prepare diazonium salt, and slowly add the obtained diazonium salt solution dropwise into a three-necked flask containing hydrogen bromide solution and cuprous bromide After that, the temperature was raised to 100°C to react for 10-12 hours, the material was discharged in deionized water, washed 3-4 times, and recrystallized with ethanol and water to obtain the product 4,4'-dibromo-3,3'-biphenol (Product B). In the reaction, the molar ratio of 3,3'-dihydroxybenzidine, sodium nitrite, and cuprous bromide is 1:...
Embodiment 2
[0046] This example takes the synthesis process of 3,3'-bis(3,4-dicarboxyphenoxy)-4,4'-diphenylethynyl biphenyl dianhydride and diamine to synthesize linear polyimide as an example Be explained.
[0047] Its synthetic route is as follows:
[0048]
[0049]
[0050] Taking 3,3'-bis(3,4-dicarboxyphenoxy)-4,4'-diphenylethynyl biphenyl dianhydride and biphenyldiamine copolymerization as an example, 3,3'-bis(3 ,4-dicarboxyphenoxy)-4,4'-diphenylethynyl biphenyl dianhydride, biphenyl diamine and m-cresol were added to the three-necked flask, reacted at 70-80°C for 10-12 hours, and heated to 170 React at ~180 ° C for 20 ~ 24 hours, discharge in absolute ethanol, wash with ethanol 4 to 5 times, and vacuum dry in a vacuum oven at 100 ° C.
[0051] The above-mentioned diamines include the following: biphenylenediamine, p-phenylenediamine, 3,3'-dihydroxybenzidine, 2,2'-bis(3-amino-4hydroxyphenyl)hexafluoropropane, 2,2 '-bis(3-amino-4-hydroxyphenyl)propane, 2,2'-bis(3-amino-4-hydr...
Embodiment 3
[0053] In the present invention, 3,3'-bis(3,4-dicarboxyphenoxy)-4,4'-diphenylethynyl biphenyl dianhydride and polyamines are prepared by adjusting the feeding ratio to obtain anhydride-blocked or amino-blocked terminal hyperbranched polyimide.
[0054] Its chemical reaction formula is as follows:
[0055]
[0056]In this example, 3,3'-bis(3,4-dicarboxyphenoxy)-4,4'-diphenylethynyl biphenyl dianhydride, 5,10,15,20-tetrakis(4-aminobenzene Base) porphyrin is an example for the synthesis of amino-terminated hyperbranched polyimide materials from raw materials, and its specific experimental steps are as follows:
[0057] (1) Add 5,10,15,20-tetrakis(4-aminophenyl)porphyrin dissolved in N-methylpyrrolidone (NMP) , slowly drop the solution of 3,3'-bis(3,4-dicarboxyphenoxy)-4,4'-diphenylethynyl biphenyl dianhydride dissolved in N-methylpyrrolidone (NMP) into the three-necked flask , react at room temperature for 10-12 hours, add 2-2.5mL isoquinoline into the there-necked flask, h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com