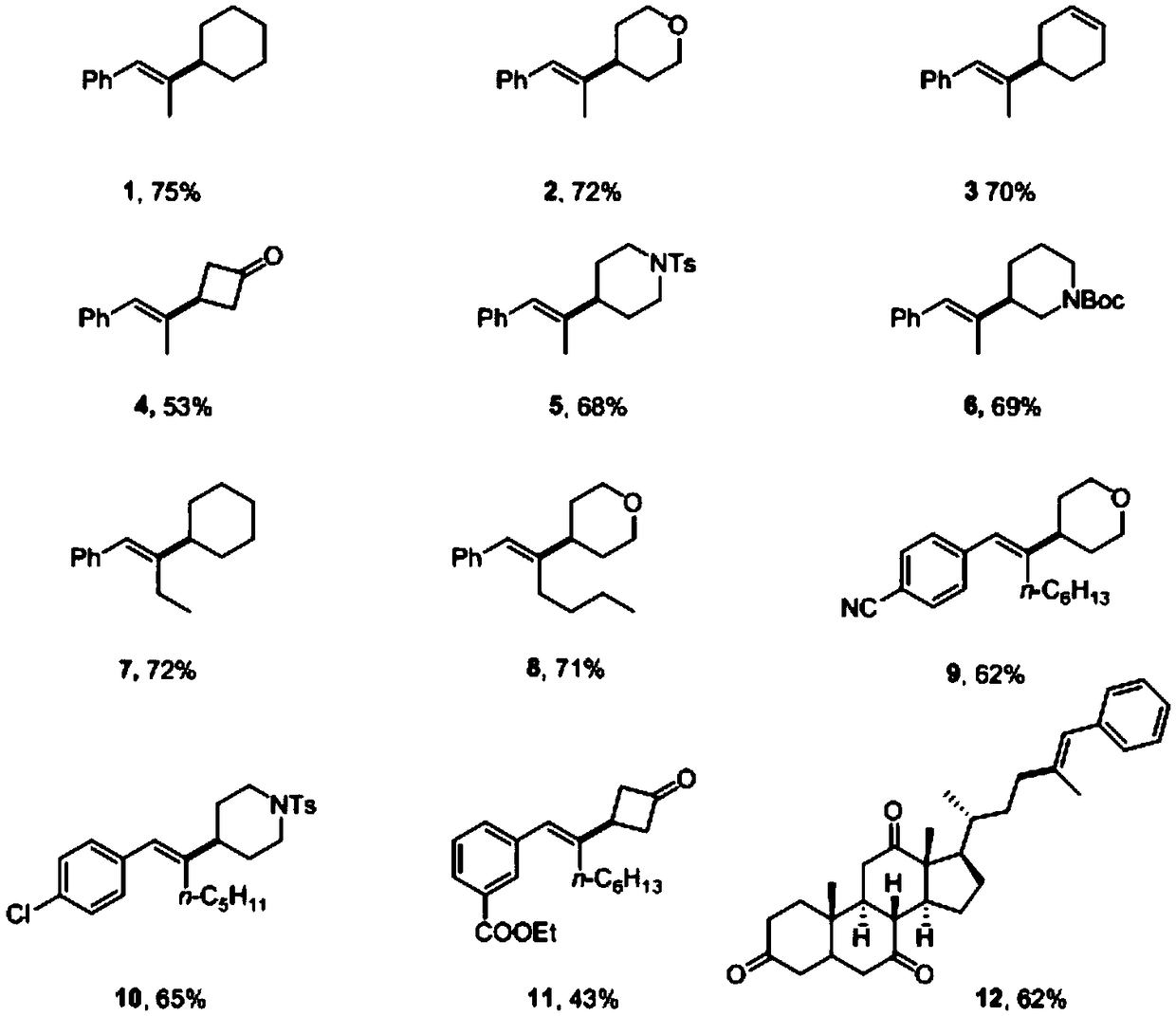
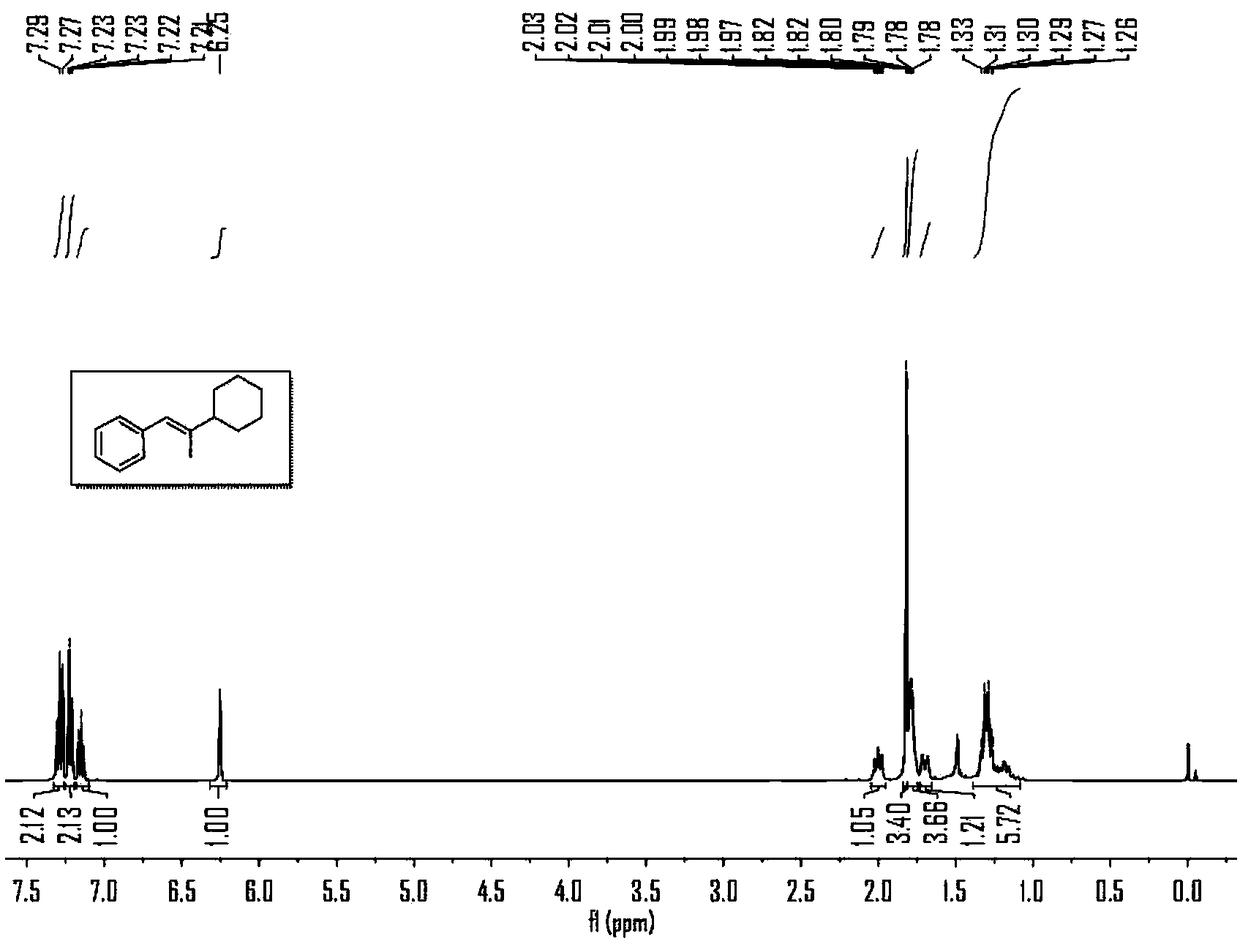
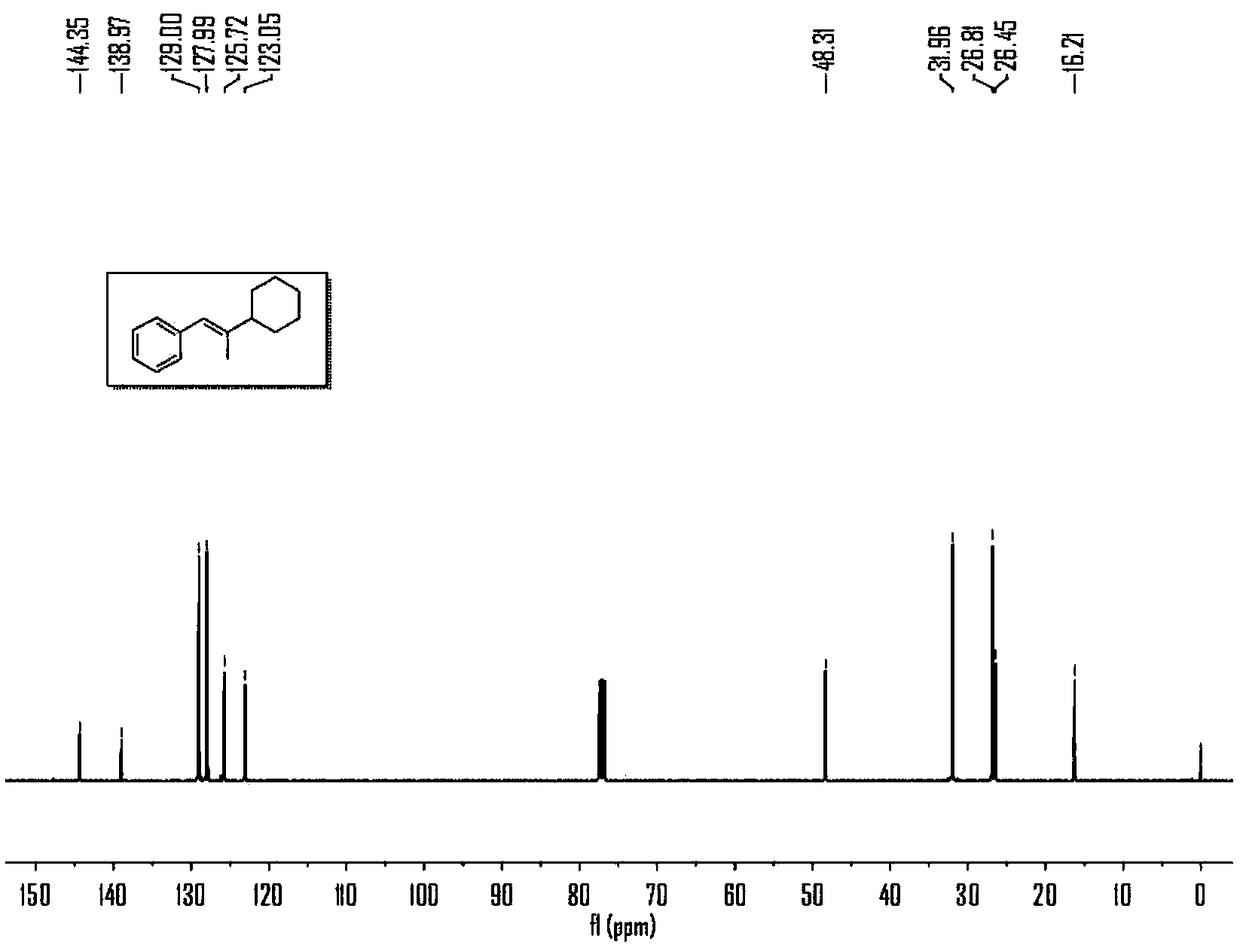
Synthesizing method for preparing trisubstituted olefin by decarboxylation reaction
A technology of decarboxylation reaction and synthesis method, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, etc., to achieve the effects of high regio and stereoselectivity, wide sources, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the reaction formula of this embodiment is as follows:
[0044]
[0045] (1) Under air, nickel(II) diethylene glycol dimethyl ether complex (12mol%), 4,4'-di-tert-butyl-2,2'-bipyridine (15mol%), ring Hexyl carboxylate (1eq), calcium acetate (3eq) was added to a sealed reaction tube with a branched tube containing magnetons, and the reaction tube was pumped three times with argon. Under the protection of argon, add 1mL N,N-dimethylacetamide to the reaction tube, stir at room temperature for 5 minutes, then add phenylpropyne (2.5eq) and (Me 2 SiH) 2 O (180 μL) was added to the reaction solution, the stopper was tightly plugged, and placed in an oil bath at 40° C. and stirred for 4 hours.
[0046] (2) Add ethyl acetate to the material obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0047] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crud...
Embodiment 2
[0049] The reaction formula of this embodiment is as follows:
[0050]
[0051] (1) Under air, nickel(II) diethylene glycol dimethyl ether complex (12mol%), 4,4'-di-tert-butyl-2,2'-bipyridine (15mol%), four Hydropyranocarboxylate (1eq) and calcium acetate (3eq) were added to a branched, sealed reaction tube containing magnetons, and the reaction tube was purged with argon three times. Under the protection of argon, add 1mL N,N-dimethylacetamide to the reaction tube, stir at room temperature for 5 minutes, then add phenylpropyne (2.5eq) and (Me 2 SiH) 2 O (180 μL) was added to the reaction solution, the stopper was tightly plugged, and placed in an oil bath at 40° C. and stirred for 4 hours.
[0052] (2) Add ethyl acetate to the material obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0053] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, and t...
Embodiment 3
[0055] The reaction formula of this embodiment is as follows:
[0056]
[0057] (1) Under air, nickel(II) diethylene glycol dimethyl ether complex (12mol%), 4,4'-di-tert-butyl-2,2'-bipyridine (15mol%), ring Hexenyl formate (1 eq) and calcium acetate (3 eq) were added to a sealed reaction tube with a branched tube containing magnets, and the reaction tube was purged with argon three times. Under the protection of argon, add 1mL N,N-dimethylacetamide to the reaction tube, stir at room temperature for 5 minutes, then add phenylpropyne (2.5eq) and (Me 2 SiH) 2 O (180 μL) was added to the reaction solution, the stopper was tightly plugged, and placed in an oil bath at 40° C. and stirred for 4 hours.
[0058] (2) Add ethyl acetate to the material obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and keep the organic phase.
[0059] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com