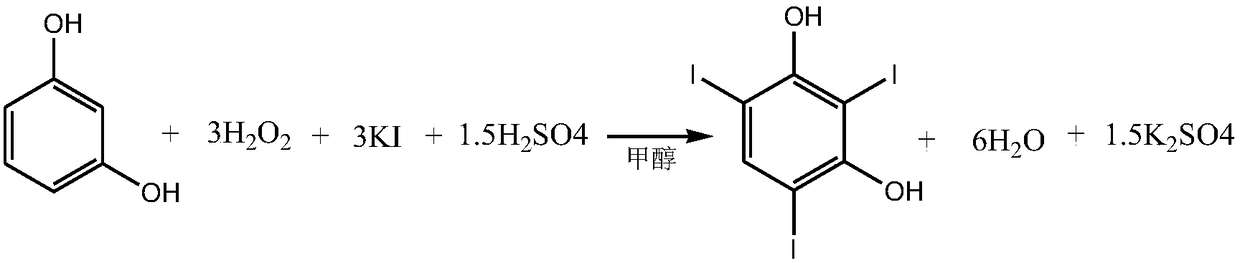
Preparation method of 2,4,6-triiodoresorcinol
A technology for triiodoresorcinol and resorcinol, which is applied in the field of preparation of iodophenol, can solve the problems of adding strong base yield, complex synthesis process route, etc., and achieves green reaction, process safety and controllability, and yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A kind of preparation method of 2,4,6-triiodoresorcinol, comprises the following steps:
[0038] (1) Dissolve 20 millimoles of concentrated sulfuric acid in 150 milliliters of methanol to obtain methanolic sulfuric acid solution A;
[0039] (2) 10 mmoles of resorcinol and 30 mmoles of potassium iodide are added in batches to solution A under stirring, then heated to 50° C., stirring and dripping 60 mmoles of a mass fraction of 30% hydrogen peroxide solution, After the dropwise addition, react at 50-54°C for 2 hours;
[0040] (3) Cool down to room temperature, vacuum precipitation under stirring, pour the residual liquid into cold water at 5-10°C, and precipitate 4.84 grams of crude product;
[0041] (4) Recrystallized from tetrachloroethylene to obtain white crystal 2,4,6-triiodoresorcinol.
[0042] (5) The conversion rate of the whole reaction is 100%. The final product yield was 96%.
Embodiment 2
[0044] A kind of preparation method of 2,4,6-triiodoresorcinol, comprises the following steps:
[0045] (1) Dissolve 20 millimoles of concentrated sulfuric acid in 100 milliliters of methanol to obtain methanolic sulfuric acid solution A;
[0046](2) 10 mmoles of resorcinol and 30 mmoles of potassium iodide are added in batches to solution A under stirring, then heated to 50° C., stirring and dripping 60 mmoles of a mass fraction of 30% hydrogen peroxide solution, After the dropwise addition, react at 50-54°C for 1 hour;
[0047] (3) Cool down to room temperature, vacuum precipitation under stirring, pour the residue into cold water at 5-10°C, and precipitate 4.82 grams of crude product;
[0048] (4) Recrystallized from tetrachloroethylene to obtain white crystal 2,4,6-triiodoresorcinol.
[0049] (5) The conversion rate of the whole reaction is 99%. The final product yield was 93.2%.
Embodiment 3
[0051] A kind of preparation method of 2,4,6-triiodoresorcinol, comprises the following steps:
[0052] (1) Dissolve 20 millimoles of concentrated sulfuric acid in 200 milliliters of methanol to obtain methanolic sulfuric acid solution A;
[0053] (2) 10 mmoles of resorcinol and 30 mmoles of potassium iodide are added in batches to solution A under stirring, then heated to 50° C., stirring and dripping 60 mmoles of a mass fraction of 30% hydrogen peroxide solution, After the dropwise addition, react at 50-54°C for 3 hours;
[0054] (3) Cool down to room temperature, vacuum precipitation under stirring, pour the residue into cold water at 5-10°C, and precipitate 4.85 grams of crude product;
[0055] (4) Recrystallized from tetrachloroethylene to obtain white crystal 2,4,6-triiodoresorcinol.
[0056] (5) The conversion rate of the whole reaction is 100%. The final product yield was 94.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com