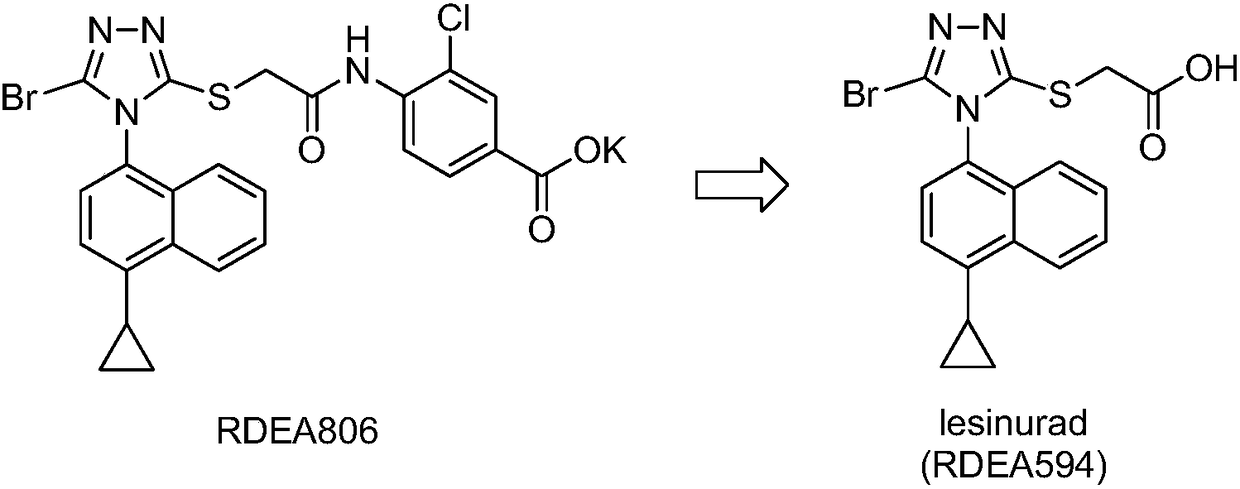
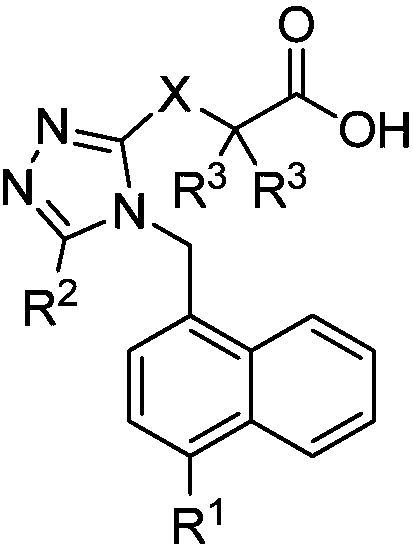
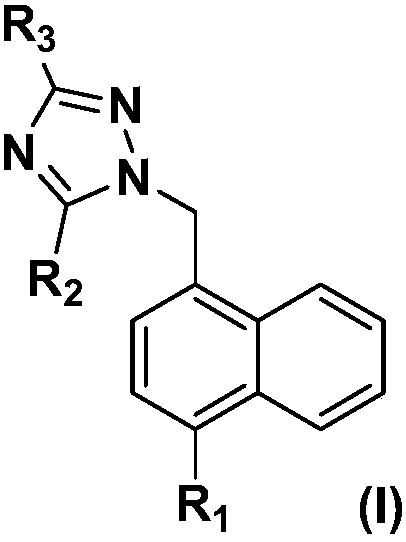
URAT1 (uric acid transporter 1) inhibitors, and preparation method and application thereof
A technology of I-A and halogenating agent, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of high incidence of side effects, decreased drug efficacy, weak selectivity and inhibitory strength, etc., and achieve strong The effect of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of Compounds I-A-1 and I-B-1
[0041]
[0042]
[0043] Step 1. Synthesis of compound IV-1
[0044] Compound II-1 (8 g, 0.08 mol) and methyl bromoacetate (III-1, 16 g, 0.10 mol) were added to tetrahydrofuran (500 ml) while stirring at room temperature. After the addition was complete, the temperature of the system was lowered to 0°C. At this time, triethylamine (20ml) was added dropwise to the system. After the dropping was completed, the system was heated up to 60° C. and stirred for 1 h, then lowered to room temperature and continued to stir for 12 h. At this time, TLC showed that the reaction was complete.
[0045] The reaction solution was filtered, the filtrate was evaporated to remove the solvent, ethyl acetate (100ml) was added to the residue and stirred at room temperature for 3h, filtered, the filter cake was collected, and air-dried at 40°C to obtain compound IV-1, a white solid, 11.3g, yield 82%. Melting point 90°C; 1 H NMR (DMSO-d6, 400 ...
Embodiment 2-35
[0065] As shown in Table 1, with reference to the method of Example 1, the following compounds with the structure of general formula (I) were synthesized.
[0066] Table 1
[0067]
[0068]
[0069]
[0070]
[0071]
[0072]
[0073]
[0074]
Embodiment 36
[0076] From compound I-A-1, its sodium salt I-A-1-S was synthesized.
[0077]
[0078] Dissolve compound I-A-1 (0.5g, 1.2mmol) in methanol (15ml), stir at room temperature, slowly add a solution prepared by NaOH (48mg, 1.2mmol) and water (1.0ml), after the addition is complete, the reaction mixture is Stirring was continued for 10 min at room temperature.
[0079] The reaction mixture was evaporated to dryness on a rotary evaporator, and the residue obtained was dissolved with methanol (10ml×2) and then evaporated to dryness to remove water in the residue, and the residue obtained was further dried in a water bath at 35°C on a vacuum oil pump After 12 hours, the sodium salt I-A-1-S of compound I-A-1 was obtained as a white solid, 0.507 g, with a yield of 96.0%. 1 H NMR (DMSO-d6, 400MHz), δ8.26-8.28(m, 1H), 7.92(s, 1H, J=8.0Hz), 7.75-7.81(m, 2H), 7.06(d, 1H, J= 8.0Hz), 5.91(s,2H), 3.95(s,2H), 3.,6(s,3H), 1.57(m,1H)0.88-0.98(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com