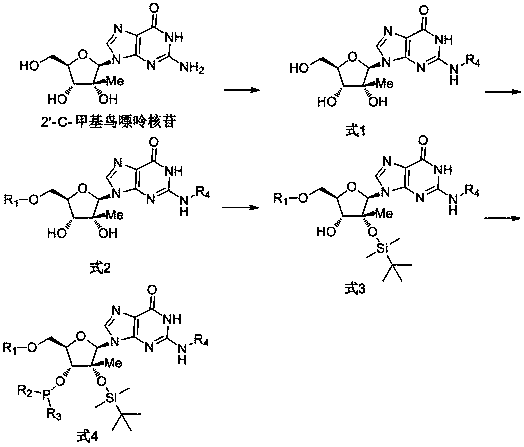
2'-C-methyl guanosine phosphoramidite monomer and synthesis method thereof
A technique for the synthesis of methylguanosine phosphoramidites and methods, which is applied in the fields of chemical instruments and methods, biochemical equipment and methods, and the preparation of sugar derivatives. Modify RNA technology development and other issues to achieve the effect of simple operation, low cost and easy cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] N 2 - Synthesis of isobutyryl-2'-C-methylguanosine:
[0035]
[0036]In a 250ml two-necked bottle, add 75ml pyridine and 3.0g (10mmol) 2'-C-methylguanosine (self-made and commercialized) respectively, and slowly drop in 8.1g (75mmol) trimethyl chloride under the condition of nitrogen protection Silane, then stirred at 25°C for 2 hours. Under nitrogen protection and 0 DEG C condition, slowly dropwise add 2.1g (20mmol) isobutyryl chloride, then 25 DEG C of stirring reaction 3 hours. Cool to 0°C, add dropwise 10ml of water, then stir the reaction at 25°C for 20 minutes, add dropwise 25ml of saturated NH 4 OH aqueous solution, then stirred the reaction at 25°C for 30 minutes. Add 150ml of water and 100ml of dichloromethane, stir for 10 minutes, let stand for liquid separation, spin dry the solvent, and then recrystallize with 5ml of water to obtain 3.0g N 2 -Isobutyryl-2'-C-methylguanosine, yield 82%.
Embodiment 2
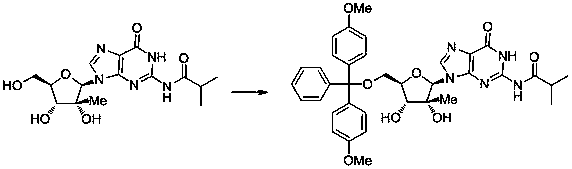
[0038] 5'-O-(4,4-Dimethoxytrityl)-N 2 - Synthesis of isobutyryl-2'-C-methylguanosine:
[0039]
[0040] In a 250ml single-necked bottle, add 1.8g (5mmol) N 2 -Isobutyryl-2'-C-methylguanosine, after azeotropic removal of water with pyridine, add 50ml of pyridine, then slowly add 1.7g (5mmol) 4,4'-bismethoxytrityl chloride dropwise 10ml of pyridine solution, then stirred at 25°C for 5 hours. Then slowly add 50ml of 5% sodium bicarbonate aqueous solution dropwise, extract three times with ethyl acetate (50ml x 3), dry with anhydrous sodium sulfate, spin the solvent, and then use 20ml (ethyl acetate / petroleum ether=3:1) Recrystallization gave 2.5 g of 5'-O-(4,4-dimethoxytrityl)-N 2 -Isobutyryl-2'-C-methylguanosine, yield 75%.
Embodiment 3
[0042] 5'-O-(4,4-dimethoxytrityl)-2'-O-[(tert-butyl)dimethylsilyl]-N 2 - Synthesis of isobutyryl-2'-C-methylguanosine:
[0043]
[0044] In a 250ml two-necked bottle, add 50mlTHF, 50ml pyridine, 0.9g (5mmol) silver nitrate solid and 3.3g (5mmol) 5'-O-(4,4-dimethoxytrityl)-N 2 -Isobutyryl-2'-C-methylguanosine, slowly dropwise add 0.8g (5mmol) of tert-butyldimethylsilyl chloride in pyridine (concentration 1mol / L) under nitrogen protection and ice bath conditions. After continuing stirring for 10 minutes under ice-bath conditions, the mixture was returned to room temperature and stirred for 5 hours. After the TLC plate detected that the reaction was complete, a saturated sodium bicarbonate solution was added in an ice bath until no bubbles emerged, and stirring was continued for 5 minutes. Extracted 3 times with dichloromethane (100mlX3), combined the organic phases, dried over anhydrous sodium sulfate, spin-dried the solvent, loaded the sample by dry method, and separated b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com