Hydrogen peroxide-responsive targeted drug-loaded nanomaterial and preparation method
A drug-loaded nano-hydrogen peroxide technology, applied in the field of nano-materials, can solve problems such as the lack of application of nano-drug-loaded systems, and achieve the effect of improving drug availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the synthesis of polyhydroxyethyl methacrylate (PHEMA)
[0025] Poly(hydroxyethylmethacrylate) (PHEMA) was synthesized by ATRP method. 2-Bipyridine (0.06g), CuCl (0.28g), 4mL N,N'-dimethylformamide, 4mL formamide were successively added to a branched bottle that was baked several times, vacuumed, and filled with argon. hydroxyethyl acrylate and 0.15 mL 2-bromo-2 methacrylate. Carry out vacuum pumping and argon filling operation again to ensure that the reaction system is in an oxygen-free state. Place the check bottle in an oil bath at 70°C for 6 hours. After the reaction is terminated, dissolve the mixture in methanol and use Al 2 o 3 The neutral column removes impurities, and the filtrate is concentrated by rotary evaporation, and precipitated in ether, and then centrifuged, dried and other post-treatments to obtain white powder PHEMA.
Embodiment 2
[0026] Example 2: Synthesis of Hydrogen Peroxide Responsive Simvastatin Polymer (PHEMA-Sim)
[0027] First, simvastatin (1.72g) was dissolved in 20mL of anhydrous dichloromethane, and oxalyl chloride (5.02g) was dissolved in 5mL of anhydrous dichloromethane. Under the condition of ice-salt bath, drop the simvastatin solution into the oxalyl chloride solution at a rate of 1 drop per second, continue to react for 2 hours after the addition, the reaction solution gradually changes from colorless to yellow, and the reaction ends. Subsequently, the solvent and excess oxalyl chloride were removed by rotary evaporation, and 1.84 g of simvastatin acid chloride yellow powder was obtained after vacuum drying, which was re-dissolved in 20 mL of anhydrous dichloromethane. Afterwards, the PHEMA (0.9g) prepared in Example 1 was dissolved in 50mL of anhydrous dichloromethane, and under the protection of argon, the dichloromethane solution containing simvastatin acyl chloride was dropped at a...
Embodiment 3
[0028] Example 3: Synthesis of targeted drug-loaded nanoparticles (PHEMA-Sim-ISO-1) in response to hydrogen peroxide
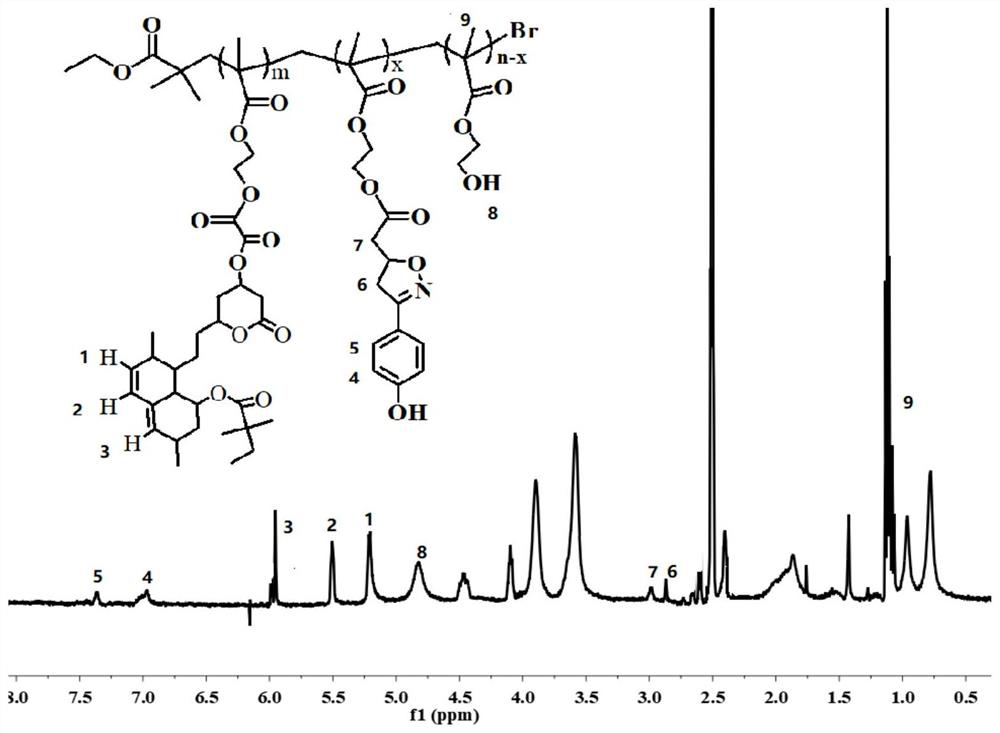
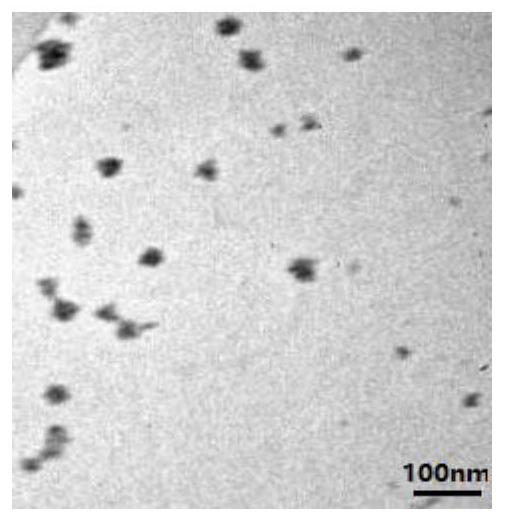
[0029] PHEAM-Sim (0.92g), ISO-1 (0.44g) and DMAP (0.12g) prepared in Example 2 were dissolved in 30mL of dichloromethane, and DCC (0.4g) was dissolved in 4mL of dichloromethane, Drop into the former mixed solution at a rate of 1 drop per second. After the dropwise addition, the reaction was continued for 24 h at room temperature. After the reaction, the target product PHEMA-Sim-ISO-1 was obtained by filtration, dialysis and drying. NMR image such as figure 1 shown, from figure 1 It can be seen that each H position and peak area of the target product have a good assignment. Transmission scanning electron microscope such as figure 2 shown, from figure 2 It can be seen that the product can form a spherical structure with a particle size of about 50 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com