Quantitative rapid deoxynivalenol detection card and detection method thereof
A technology of deoxynivalenol and detection card, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problem of affecting the accuracy and stability of test results, difficult to distinguish negative and positive test results, and difficult to be accurate in particle size deviation. Control and other issues, to achieve the effect of saving manpower and material resources, easy to distinguish, and small differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
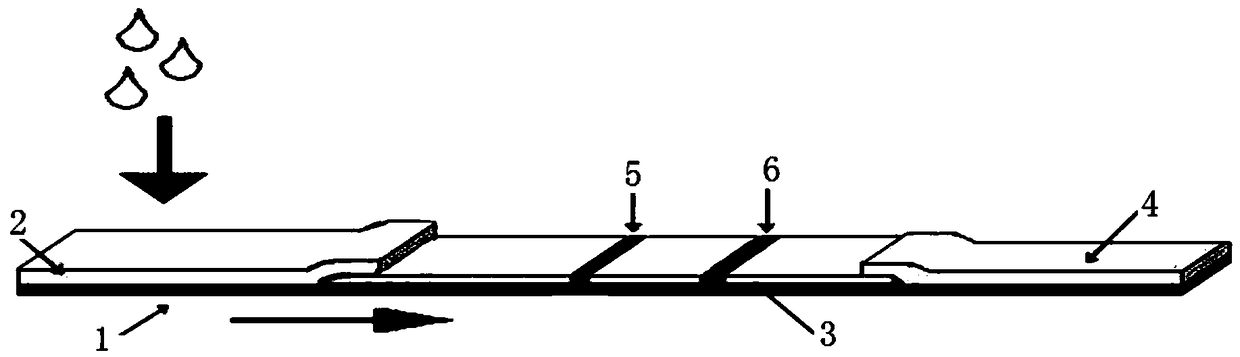
[0060] to combine figure 1 As shown, a deoxynivalenol quantitative rapid detection card provided in this embodiment includes a sample pad 2, a nitrocellulose membrane 3 and a water-absorbing pad 4 arranged on the base plate 1 in sequence, and does not include colloidal gold Release pad of labeled antibody or monoclonal antibody with detection line (T line) coated with deoxynivalenol hapten-carrier protein coupling agent (DON-BSA) on the nitrocellulose membrane 3 5 and the quality control line (C line) 6 coated with goat anti-mouse antibody, the sample solution to be tested is mixed and incubated with latex microspheres coupled with DON antibody before being dropped onto the sample pad.
[0061] In this embodiment, the coating amounts of the detection line 5 and the quality control line 6 are respectively 0.1 mg / mL and 0.4 mg / mL, and the nitrocellulose membrane 3 is After the coating of the quality control line 6, it was dried overnight at 37°C.
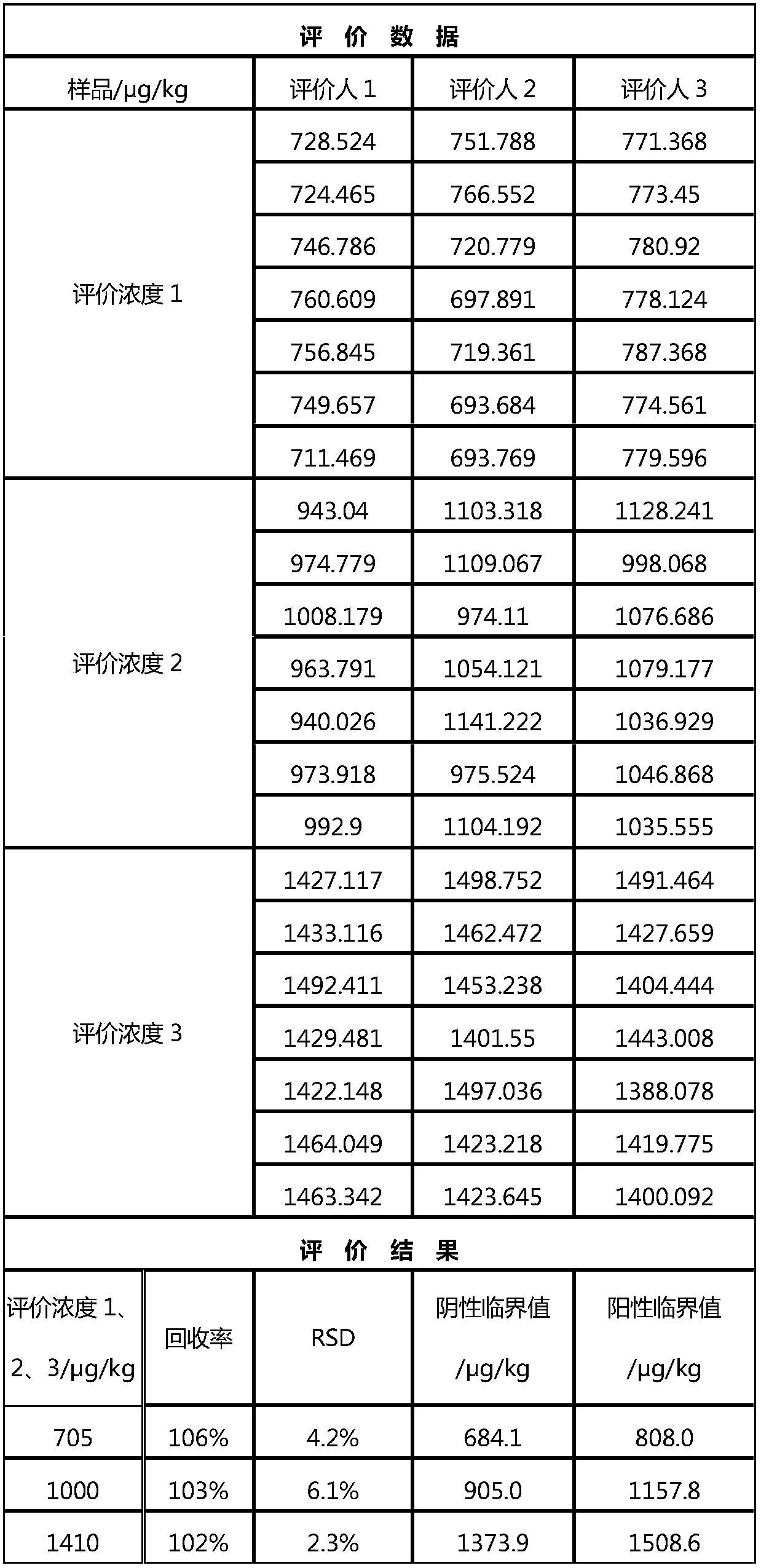
[0062] In this embodiment, ...
Embodiment 2
[0074] The deoxynivalenol quantitative rapid detection card described in this example is the same as in Example 1, except that the DON antibody is a monoclonal antibody, and the monoclonal antibody is prepared by the following method:
[0075] (1) Preparation of immunogen
[0076] Dissolve 8 mg of deoxynivalenol hapten in 0.8 mL of DMF, then add 20 mg of EDC fully dissolved in 0.2 mL of water, and stir at room temperature for 24 hours to obtain reaction solution A; weigh 36 mg of OVA was fully dissolved in 3 mL of CB with a concentration of 0.1 mol / L and a pH of 9.6 to obtain a protein solution A; the reaction solution A was slowly added dropwise to the protein solution A, and stirred at room temperature for 24 Hours, dialyze with PBS with a concentration of 0.01mol / L at 4°C for 3 days and change the dialysate 3 times a day to obtain the immunogen;
[0077] (2) immunity
[0078] Immunizing Balb / c mice with the immunogen obtained in step (1), the immunological dose is 100 μg / ...
Embodiment 3
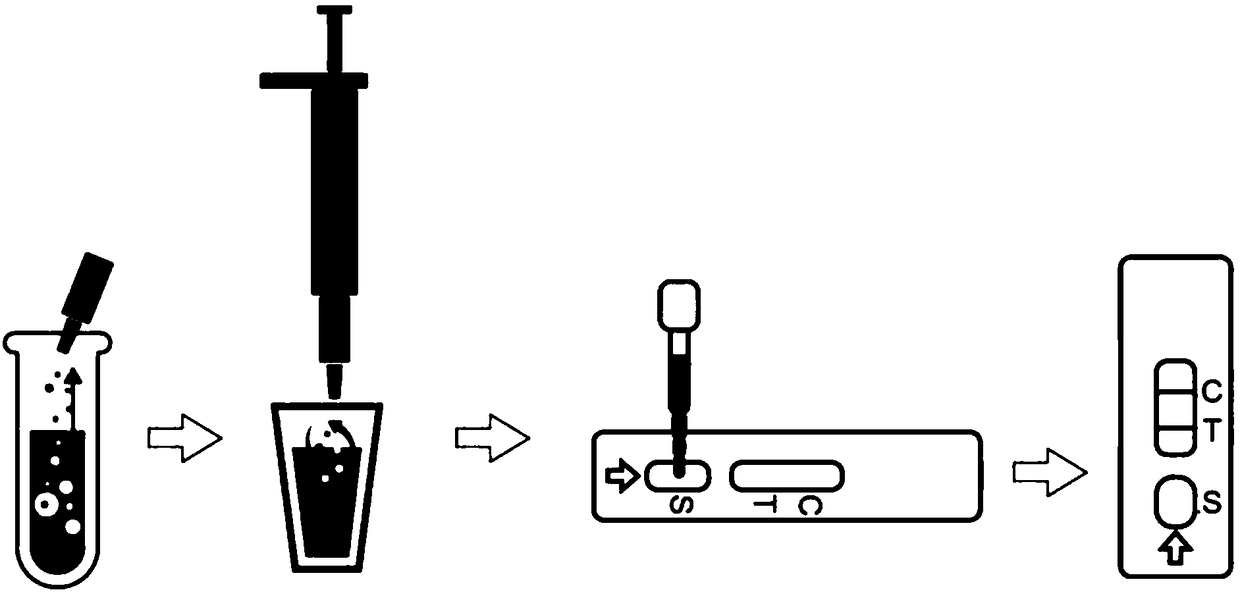
[0086] to combine figure 2 As shown, the present embodiment provides a kind of detection method of deoxynivalenol quantitative rapid detection card described in embodiment 1, comprises the steps:
[0087] (1) Preheating: turn on the incubator switch and raise the temperature to 45±1°C before testing;
[0088] (2) Labeled samples: According to the number of samples to be tested, take out the corresponding number of latex microspheres coupled with DON antibody and the test card that have been lyophilized and coated, and mark on the test card to distinguish the sample solution to be tested
[0089] (3) Liquid addition: use a pipette to draw 120ul of the sample solution to be tested, drop it vertically into the latex microspheres coupled with DON antibody that have been lyophilized and coated, and repeatedly pipette for more than 10 times until the mixture is uniform;
[0090] (4) Incubation: place the detection card and the solution obtained in step (2) on the incubator, and inc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com