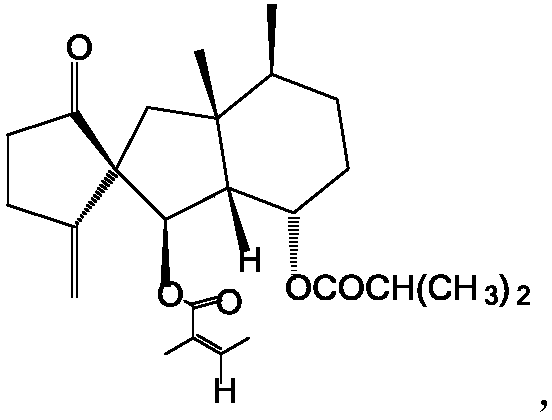
Fukinanclide K-containing antifungal composition for external use
A technology of butterbur lactone and external composition, which is applied in the direction of medical preparations containing active ingredients, antifungal agents, and medical preparations without active ingredients, which can solve the problems of stimulating drug resistance of pathogenic bacteria and achieve Good antibacterial effect, improved therapeutic effect, high therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
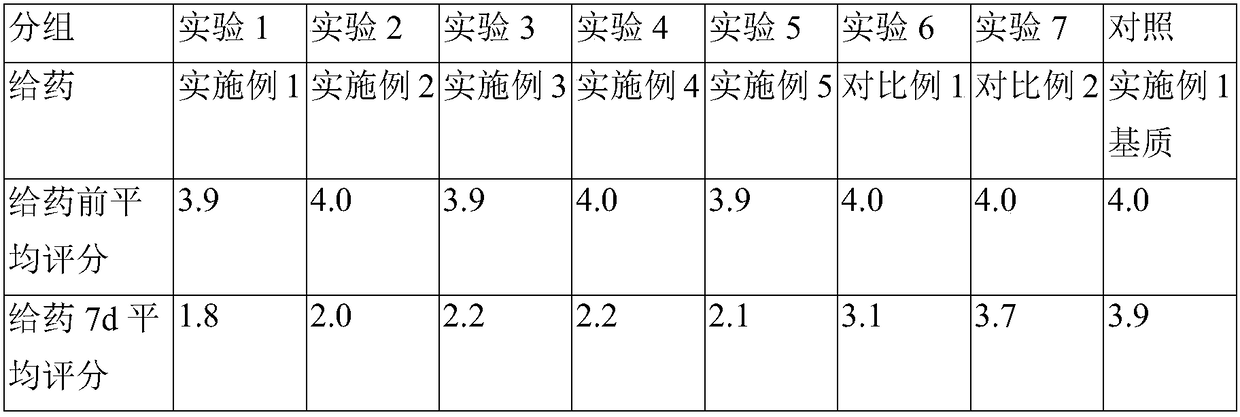
Embodiment 1
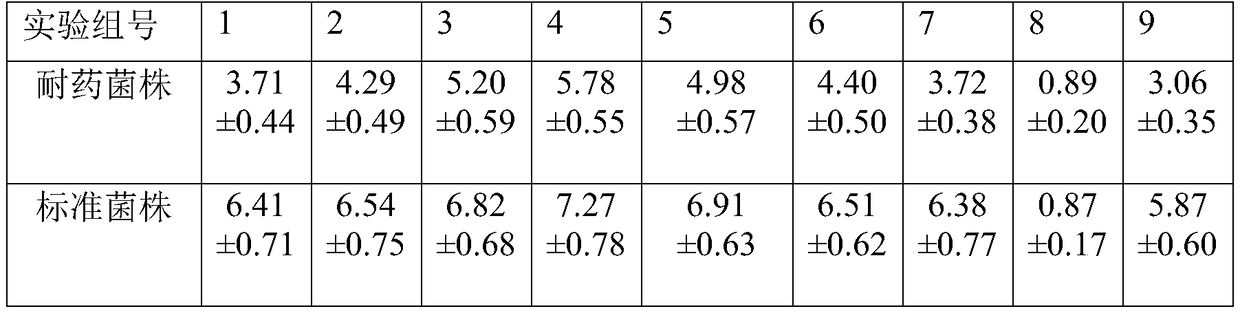
[0014] Pharmacological Example 1. Preparation of drug-resistant strains
[0015] Preparation
[0016] 1) Standard strain Trichophyton rubrum (No.: ATCC228188) was used as the standard strain, and the standard strain of Trichophyton rubrum was inoculated on potato dextrose agar (PDA) medium, and activated in an incubator at 28°C for 7 to 14 days , select the colonies with good growth status and no pollution as the test strains, select the colonies with good growth status and no pollution as the test strains, drop an appropriate amount of 0.85% sterile saline on the colonies, and gently repeat with a sterile cotton swab Scrape the surface of the colony to mix the spores well with the normal saline. Filter the bacterial suspension with two layers of gauze into a centrifuge tube, and then place it in a vortex shaker for about 15s. Count with a hemocytometer, and adjust the bacterial concentration to 2-6×10 with sterile saline. 5 cfu / mL, and finally diluted 10 times with PRMI-16...
Embodiment 2
[0019] Pharmacological Example 2. In vitro antibacterial effect test
[0020] 1. Preparation of culture medium
[0021] Potato dextrose agar medium (PDA), the mass fraction is 20% potato, 2% glucose, and 1.8% agar. Add 5ml of medium to each test tube to prepare a slant medium; add 20ml of medium to a plate with a diameter of 90mm to prepare a plate medium
[0022] 2. Experimental method
[0023] 2.1 Treatment of strains
[0024] 1. The artificially induced drug-resistant Trichophyton rubrum strain and standard strain prepared in Pharmacological Example 1 were transferred to the PDA medium activation strain on a plate using a sterilization inoculation loop respectively, placed in a 28°C constant temperature incubator, and cultivated for 7 days.
[0025] 2.2 Making bacterial suspension
[0026] Pick an appropriate colony mass and dissolve it in 1 mL of 0.9% sterile saline with Tween 80 added. Shake with a micro-shaker for 2 minutes, fully shake to wash out the spores, and u...
Embodiment 3
[0050] Butterolide K 8.4g, bifonazole 7g
[0051] Sodium carboxymethyl cellulose 60g, glycerol 150g
[0052] Add 2g of ethyl paraben to distilled water to 1000g
[0053] Mix CMC-Na and glycerol to obtain CMC-Na slurry, dissolve ethyl paraben in hot water, then add CMC-Na slurry, stir to form gel, and then add bifonazole and bee bucket. The vegetable lactone K is dissolved in an appropriate amount of ethanol, gradually added and stirred, and the remaining amount of water is added and stirred to obtain a transparent gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com