Neural stem cell preparation used for cerebral infarction treatment and bimodally traceable by magnetic resonance and fluorescence imaging
A stem cell and host cell technology, applied in the fields of genetic engineering and biomedical engineering, can solve the problems of stem cell apoptosis, inaccurate stem cell treatment effect, low survival rate of stem cells, etc., to increase the number, improve anti-apoptosis and viability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
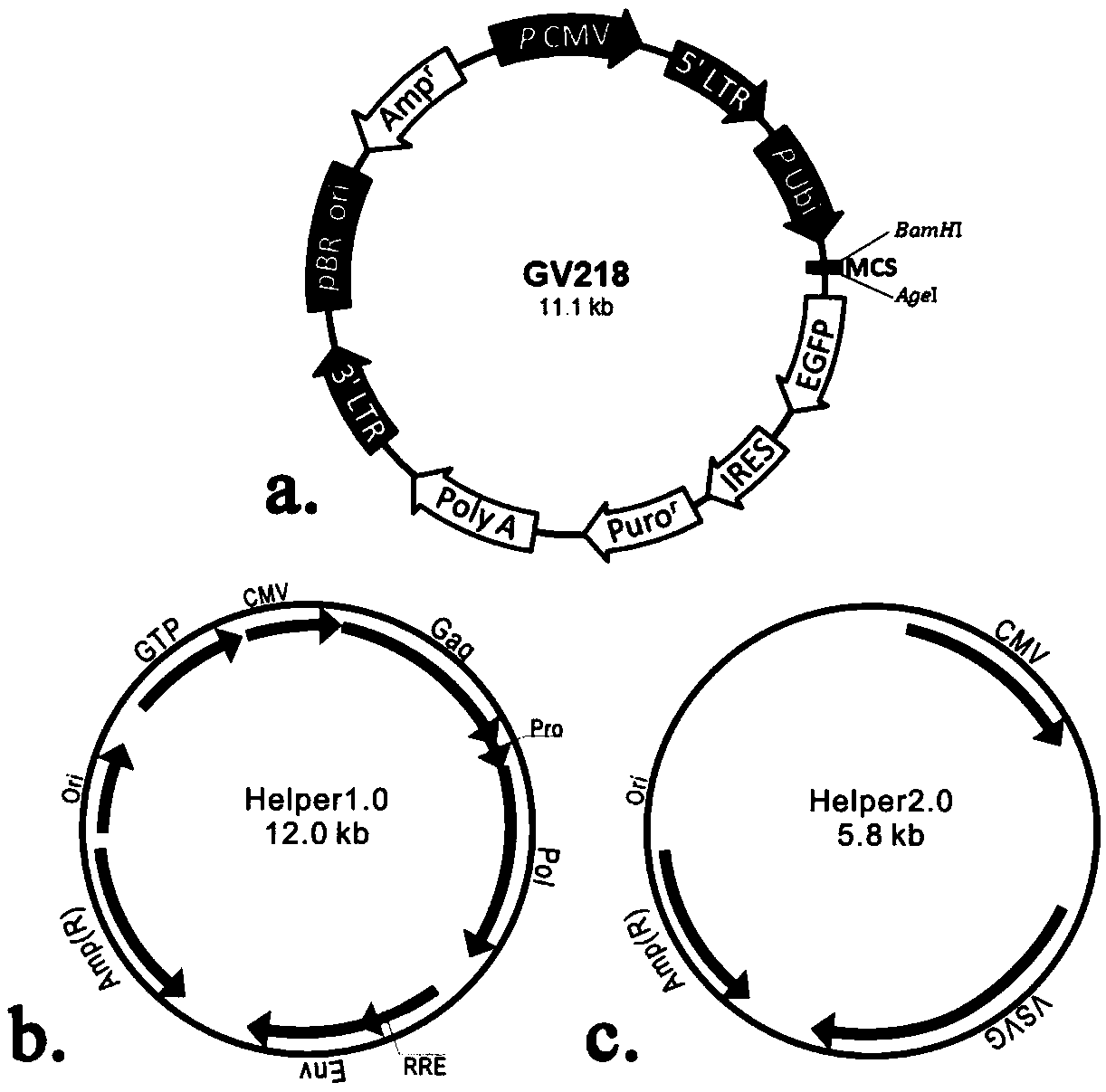
[0054] Example 1 Construction and Identification of FerritinH-T2A-Bcl2-EGFP Overexpression Lentiviral Vector
[0055] 1. Acquisition of the target gene
[0056] According to the gene sequence of rat FerritinH (NM_012848) and Bcl2 (NM_016993) in GenBank combined with the sequence of self-cleaving polypeptide T2A, the rat FerritinH-T2A-Bcl2 gene was synthesized by chemical synthesis. Design specific primers as follows:
[0057] FerritinH-T2A-Bcl2 upstream primer (SEQ ID NO: 1): FerritinH+Bcl2-P1
[0058] GAGGATCCCCGGGTACCGGTCGCCACCATGACCACCGCGTCTCCCTC
[0059] FerritinH-T2A-Bcl2 downstream primer (SEQ ID NO: 2): FerritinH+Bcl2-P2
[0060] TCACCATGGTGGCGACCGGCTTGTGGCCCAGGTATGCAC
[0061] Primers were used to amplify the FerritinH-T2A-IFNβ sequence by PCR. After the PCR product was electrophoresed on the agarose gel, the target band with a molecular weight of about 1Kb was excised for later use.
[0062] 2. Construction and sequencing of recombinant plasmids
[0063] The re...
Embodiment 2
[0070] Example 2 Determination of Optimal Transfection Conditions for NSCs by FerritinH-T2A-Bcl2-EGFP Overexpression Lentivirus
[0071] 1. Isolation and culture of NSCs
[0072] Neonatal 1-day-old SD rats were killed by necking, and the tissues around the bilateral lateral ventricles were separated under aseptic conditions, the brain tissue was cut into pieces, filtered, centrifuged, and the supernatant was discarded, resuspended and inoculated to 25 cm 2 In culture flask, 37°C, 5% CO 2 1. Cultivate under saturated humidity until neurospheres are formed. When the light transmittance of neurospheres is significantly reduced, subculture at 1:2.
[0073] 2. Determination of optimal transfection conditions for NSCs by FerritinH-T2A-Bcl2-EGFP overexpression lentivirus
[0074] Press 1×10 5 NSCs cells / well were planted in a 12-well culture plate, and 1 mL of complete medium was added to each well. When the cell confluency was about 50%, the culture medium was removed, washed wi...
Embodiment 3
[0076] Example 3 In vitro effectiveness detection of FerritinH-T2A-Bcl2-EGFP overexpression lentivirus transfected NSCs
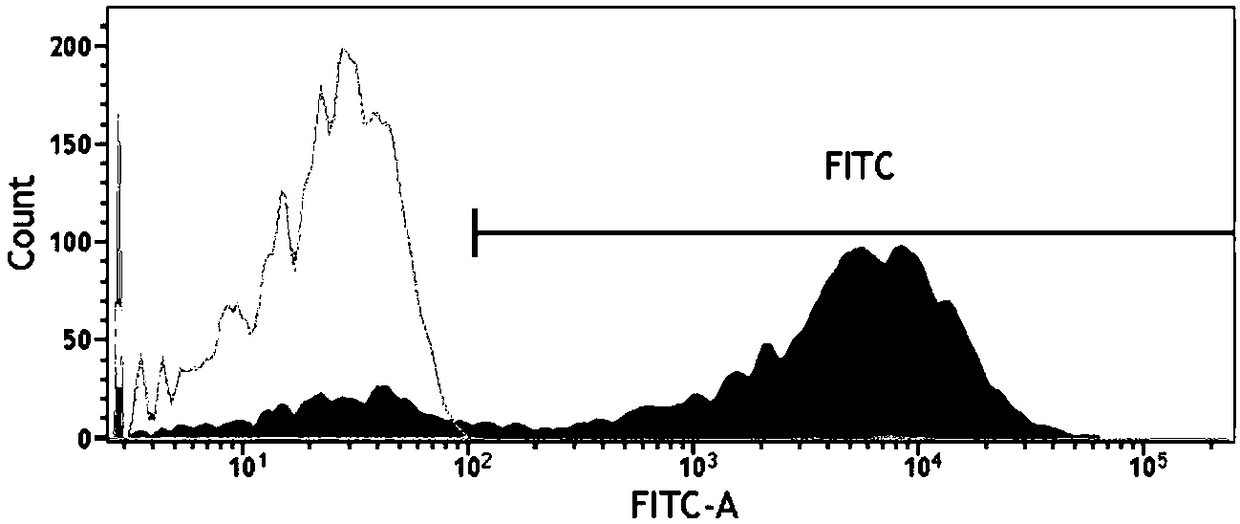
[0077] 1. Fluorescent expression rate of FTH-Bcl2-EGFP-NSCs detected by flow cytometry
[0078] NSCs by 2×10 5 Cells / well were seeded in a 6-well plate, and lentiviral transfection was performed according to the optimal transfection conditions above. After 24 hours, the virus-containing culture medium was removed, the culture medium was removed, washed with PBS, digested with trypsin, blown into a cell suspension, and counted. Take 5×10 5 After each FTH-Bcl2-NSCs, the expression rate of eGFP in transfected cells was detected by flow cytometry.
[0079] The results show that the fluorescence expression of EGFP measured by flow cytometry is shown in Figure 4 , the expression rate of EGFP was stable at about 78.62±0.29% after transfection.
[0080] 2. Real-time PCR detection of FerritinH, Bcl2 mRNA levels of NSCs after transfection
[0081] NSCs by 2×10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com