Cyclosporine conjugate and preparation method and application thereof
A technology for cyclosporine and conjugates, which is applied in the field of cyclosporine conjugates and their preparation, can solve the problems of high price, organic solvent pollution, time-consuming and other problems, save detection time, and make up for time-consuming problems. long effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
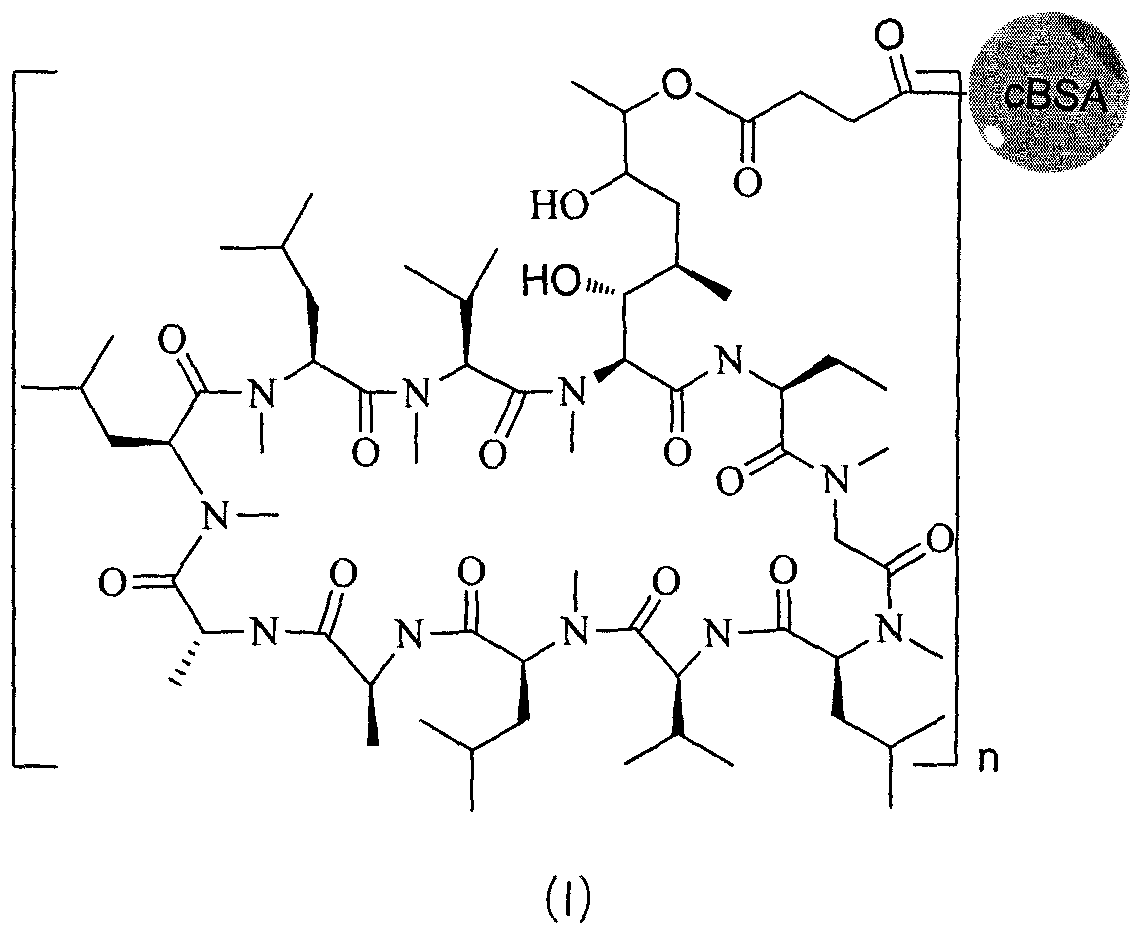
[0040] (1) Preparation of solution A: 50 mg of cyclosporine (0.042 mmol) was added to a 25 mL round bottom flask, and dissolved in 10 mL of dichloromethane. Add 14 mg m-chloroperoxybenzoic acid (MCPBA) to it, stir overnight at room temperature, then add aqueous sodium bicarbonate solution, extract with dichloromethane, combine the organic phases, and rotate under reduced pressure to obtain the product dissolved in dilute hydrochloric acid aqueous solution and stir at room temperature After 5h, adjust the pH to 7, add succinic anhydride, hydroxysuccinimide, ethyl [3-(dimethylamino) propyl] carbodiimide (EDC) at a molar ratio of 1:2 to 8:5 ~15 was dissolved in DMF-water two-phase mixed solvent, and reacted to generate active intermediates of cyclosporine analogs and ethyl[3-(dimethylamino)propyl]carbodiimide, which was solution A.
[0041](2) Preparation of cBSA: Dissolve 18.0mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.40 and a concentration of 0.01...
Embodiment 2
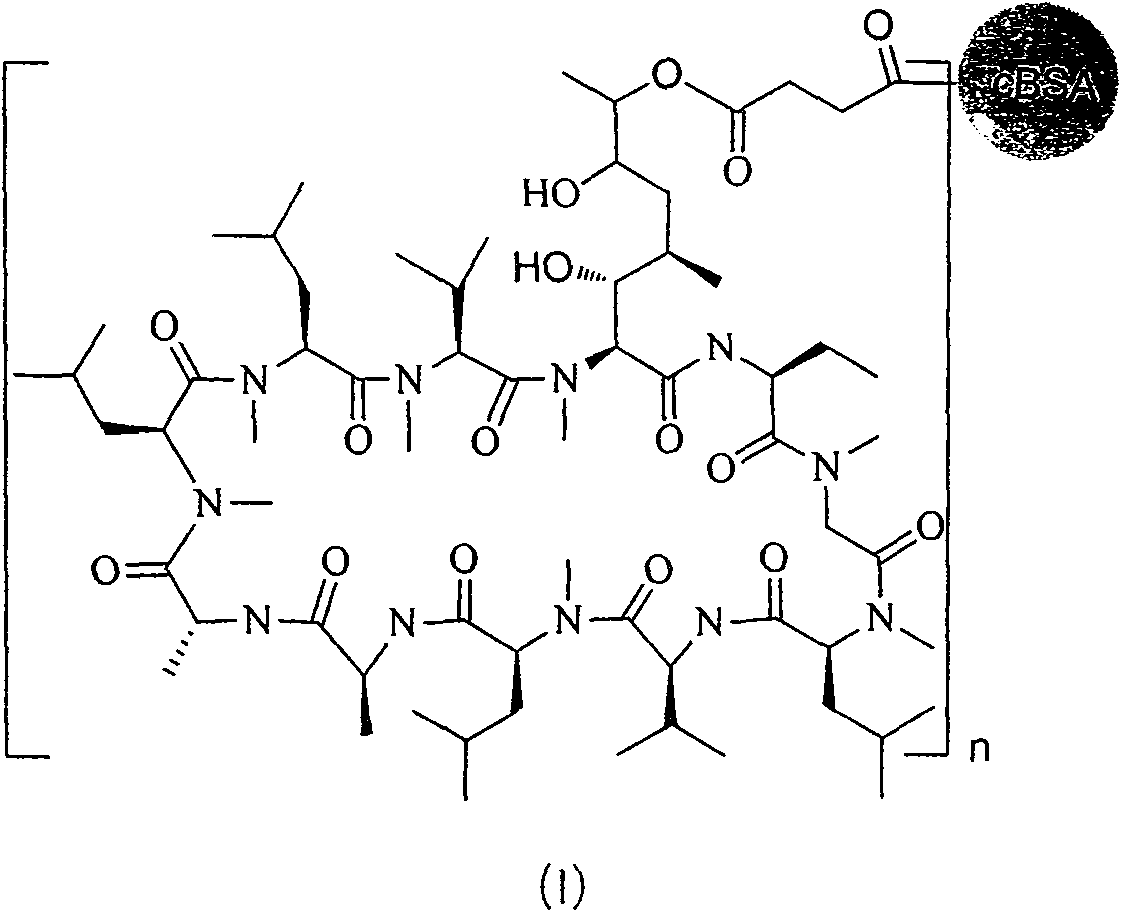
[0047] (1) Preparation of solution A: 50 mg of cyclosporine (0.042 mmol) was added to a 25 mL round bottom flask, and dissolved in 10 mL of dichloromethane. Add 14 mg m-chloroperoxybenzoic acid (MCPBA) to it, stir overnight at room temperature, then add aqueous sodium bicarbonate solution, extract with dichloromethane, combine the organic phases, and rotate under reduced pressure to obtain the product dissolved in dilute hydrochloric acid aqueous solution and stir at room temperature After 5h, adjust the pH to 7, add succinic anhydride, hydroxysuccinimide, ethyl [3-(dimethylamino) propyl] carbodiimide (EDC) at a molar ratio of 1:2 to 8:5 ~15 was dissolved in DMF-water two-phase mixed solvent, and reacted to generate active intermediates of cyclosporine analogs and ethyl[3-(dimethylamino)propyl]carbodiimide, which was solution A.
[0048] (2) Preparation of cBSA: Dissolve 18.0mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.40 and a concentration of 0.0...
Embodiment 3
[0054] Preparation, purification and detection of antibodies
[0055] 1. Antibody Preparation
[0056] The cyclosporine conjugate prepared in Example 2 above was selected as the immunogen to carry out animal immunization experiments to prepare antibodies.
[0057] Take 1ml of the solution of the conjugate of cyclosporine at 1mg / ml, add an equal volume of Freund's complete adjuvant, fully emulsify, and inject into four male healthy New Zealand white rabbits with a body weight of 2kg at multiple points subcutaneously, 1ml / Only, 15 days later, the same amount of antigen was fully emulsified with Freund's incomplete adjuvant for the second immunization. After the second immunization, a booster immunization was performed every 15 days, and the amount of antigen was halved, and a total of 5 immunizations were given. Seven days after the last immunization, blood was collected from the heart, left at room temperature for 1 hour, overnight at 0-4°C, centrifuged at 13,000 rpm for 15 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com