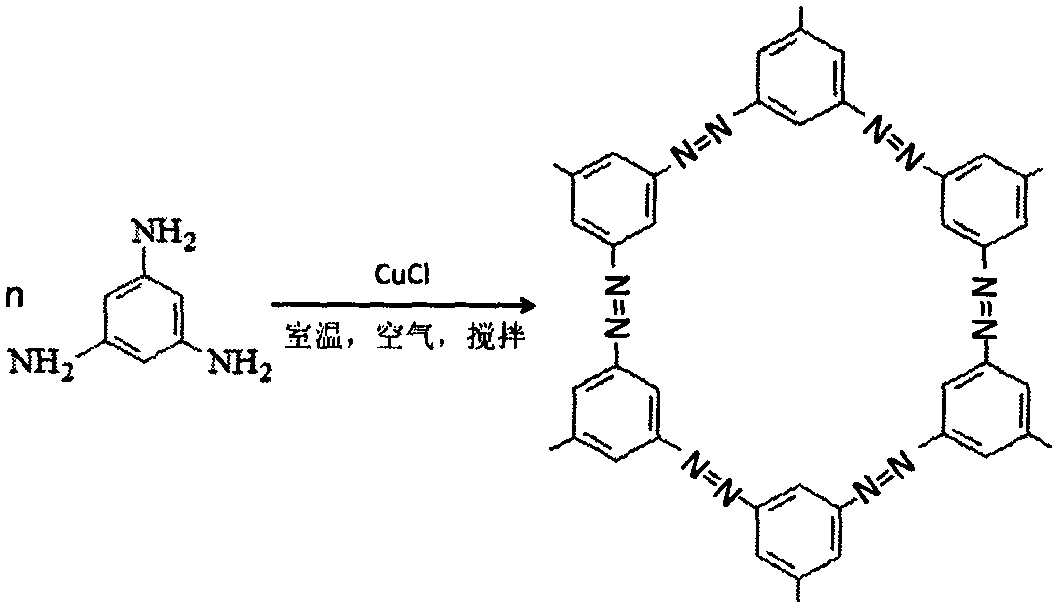
Preparation method of azo porous material/carbon nanotube composite electrode material
A carbon nanotube composite and porous material technology, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems that the template method is not suitable for large-scale production, the nitrogen atom doping process is complicated, and the doping degree is limited. Industrial batch production, high Coulombic efficiency and rate performance, simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Weigh 0.8524g of copper chloride dihydrate, place it in a 50mL polytetrafluoroethylene-lined autoclave, add 25mL of ethanol, mix well, and cover the kettle tightly.
[0027] (2) Put the autoclave in a muffle furnace and react at 160°C for 24h. Cool to room temperature, filter under reduced pressure, and dry the product in vacuum at 50°C for 12 hours. Store in a dark place under a nitrogen protection atmosphere to obtain cuprous chloride nanocrystals with a diameter of 2-10 nm.
[0028] (3) 10 mg of cuprous chloride crystal powder, 10 mg of carbon nanotubes and 0.123 g of 1,3,5-triaminobenzene were dissolved in 5.0 mL of acetonitrile, and stirred at room temperature for 10 h.
[0029] (4) The reaction mixture was taken out, washed with 25% ammonia water, water and acetone respectively, and dried and stored.
[0030] It has been determined that in the nitrogen-rich porous material / carbon nanocomposite material, the nitrogen element content is 27.1% by weight; the sp...
Embodiment 2
[0036] (1) Weigh 0.8524g of copper chloride dihydrate, place it in a 50mL polytetrafluoroethylene-lined autoclave, add 25mL of ethanol, mix well, and cover the kettle tightly.
[0037] (2) Put the autoclave in a muffle furnace and react at 160°C for 24h. Cool to room temperature, filter under reduced pressure, and dry the product in vacuum at 50°C for 12 hours. Store in a dark place under a nitrogen protection atmosphere to obtain cuprous chloride nanocrystals with a diameter of 2-10 nm.
[0038] (3) 10mg of cuprous chloride crystal powder, 10mg of carbon nanotubes and 0.249g of 2,4,6-triethylbenzene-1,3,5-trimethylamine were dissolved in 5.0mL of acetonitrile, stirred at room temperature 10h.
[0039] (4) The reaction mixture was taken out, washed with 25% ammonia water, water and acetone respectively, and dried and stored.
Embodiment 3
[0041] (1) Weigh 0.8524g of copper chloride dihydrate, place it in a 50mL polytetrafluoroethylene-lined autoclave, add 25mL of ethanol, mix well, and cover the kettle tightly.
[0042] (2) Put the autoclave in a muffle furnace and react at 160°C for 24h. Cool to room temperature, filter under reduced pressure, and dry the product in vacuum at 50°C for 12 hours. Store in a dark place under a nitrogen protection atmosphere to obtain cuprous chloride nanocrystals with a diameter of 2-10 nm.
[0043] (3) 10mg of cuprous chloride crystal powder, 10mg of carbon nanotubes and 0.249g of 2,4,6-trimethylbenzene-1,3,5-triamine were dissolved in 5.0mL of acetonitrile, stirred at room temperature 10h.
[0044] (4) The reaction mixture was taken out, washed with 25% ammonia water, water and acetone respectively, and dried and stored.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com