Synthetic method of doxofylline
A technology of doxofylline and its synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high cost and difficult availability of raw materials, and achieve the effects of short reaction time, low reaction temperature and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
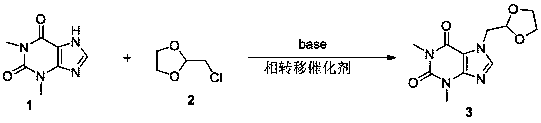
[0022] Add 10kg (55.6mol) of theophylline, 6L of acetone, 4.45kg of sodium hydroxide (111.2mol) and 0.56kg of tetrabutylammonium chloride (2mol) into the reaction tank, stir for 20 minutes and add 8.18kg of chloroacetaldehyde acetal Ethylene glycol (66.7mol), stirred and refluxed for 6h, TLC (acetone:dichloromethane=3:1) monitored the complete reaction of the raw material theophylline. After the reaction was over, the solvent was evaporated to dryness under reduced pressure, washed 3 times with saturated saline, filtered, and the filter residue was recrystallized with absolute ethanol to obtain 13.3kg (50.04mol) of product doxofylline, with a yield of 90% and a melting point of 143-145°C.
Embodiment 2
[0024] Add 10kg (55.6mol) of theophylline, 8L of dichloromethane, 6.23kg of potassium hydroxide (111.2mol) and 0.7kg of tetrabutylammonium chloride (2.5mol) into the reaction tank, stir for 20 minutes and then add 12.3kg of chlorinated Acetaldehyde ethylene glycol (100mol), stirred and refluxed for 5h, TLC (acetone: dichloromethane = 3:1) monitoring the complete reaction of the raw material theophylline. After the reaction, the solvent was evaporated to dryness under reduced pressure, washed three times with saturated saline, filtered, and the filter residue was recrystallized with absolute ethanol to obtain the product doxofylline 13kg (48.9mol), with a yield of 88% and a melting point of 143 -145°C.
Embodiment 3
[0026] Add theophylline 10kg (55.6mol), 8L DMF, 8.8kg sodium carbonate (83.4mol) and 0.56kg tetrabutylammonium chloride (2mol) into the reaction tank, stir for 20min and add 8.18kg chloroacetaldehyde acetal dropwise Diol (66.7mol), heated to 110°C and kept stirring at this temperature for 6h, TLC (acetone:dichloromethane=3:1) monitored the complete reaction of the raw material theophylline. After the reaction, the solvent was evaporated to dryness under reduced pressure, washed three times with saturated saline, filtered, and the filter residue was recrystallized with absolute ethanol to obtain the product doxofylline 13kg (48.9mol), with a yield of 88% and a melting point of 143 -145°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com