Method for constructing recombinant bacteria capable of efficiently secreting and expressing bacterial heparin hydrolase
A construction method, the technology of bacterial heparin, applied in the field of enzyme engineering, can solve the problems of intracellular expression not achieving high-efficiency secretion expression, yield not reported, etc., and achieve the effect of simple method, easy scale-up culture, and low nutritional requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Construction of recombinant Pichia pastoris producing heparan hydrolase
[0024] Construction of a genetically engineered bacterium producing heparan hydrolase:
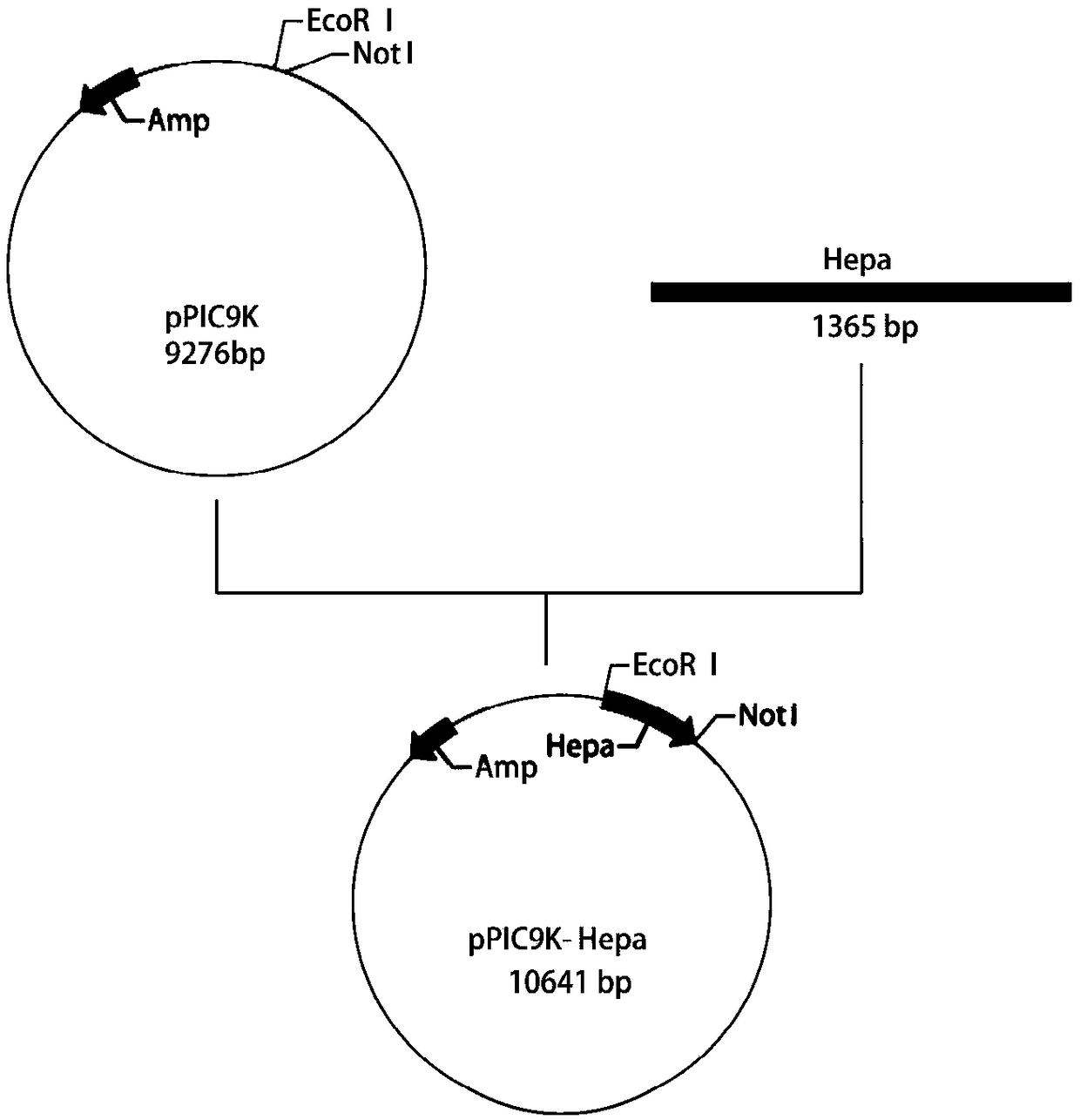
[0025] Using pPIC9K as a vector, the heparin hydrolase gene (nucleotide sequence shown in SEQ ID NO: 1) was cloned, and a 6-his tag was added after the gene ATG. Such as figure 1 As shown, the upstream primer introduces the EcoR I restriction enzyme site; the downstream primer introduces the Not I restriction enzyme site; after gene Hepa and plasmid pPIC9K are digested and purified by restriction endonuclease EcoR I and Not I, 16°C Overnight ligation was transferred into Escherichia coli JM109 for amplification, and the plasmids with correct sequencing were selected and transferred into Pichiapastoris GS115 for expression to obtain recombinant Pichia pastoris GS115 / pPIC9K-BpHEP containing the recombinant heparin hydrolase gene.
[0026] The upstream and downstream primers are:
[0027] F: GAATTCA...
Embodiment 2
[0029] Example 2: Construction of Escherichia coli recombinant bacteria producing heparan hydrolase
[0030] Construction of a genetically engineered bacterium producing heparan hydrolase:
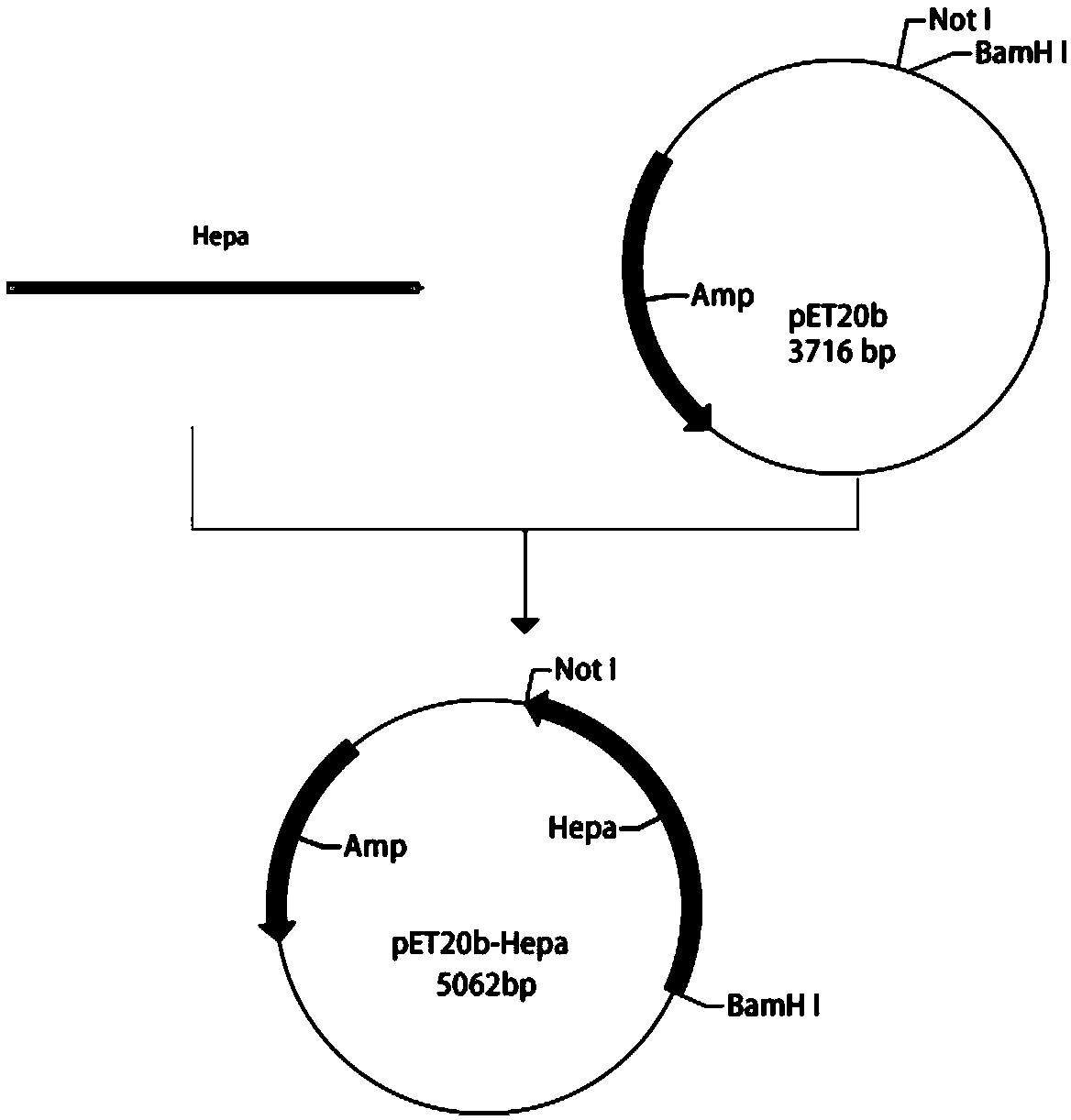
[0031] Using pET20 as a vector, the heparin hydrolase gene was cloned. Such as figure 2 As shown, the upstream primer introduces the EcoR I restriction enzyme site; the downstream primer introduces the Not I restriction enzyme site; after gene Hepa and plasmid pET20 are digested and purified by restriction endonuclease EcoR I and Not I, 16°C Overnight ligation was transferred into Escherichia coli JM109 for amplification, and the plasmids with correct sequencing were selected and transferred into BL21 for expression to obtain recombinant Escherichia coli BL21DE3 / PET20-BpHEP containing the recombinant heparin hydrolase gene.
[0032] The upstream and downstream primers are:
[0033] F: GAATTCATGCCGCAGCAGCCGCCG (SEQ ID NO: 3)
[0034] R: GCGGCCGCTTACAATTGAATAGATT (SEQ ID NO: 4)
Embodiment 3
[0035] Embodiment 3: Shake flask horizontal fermentation produces heparin hydrolase
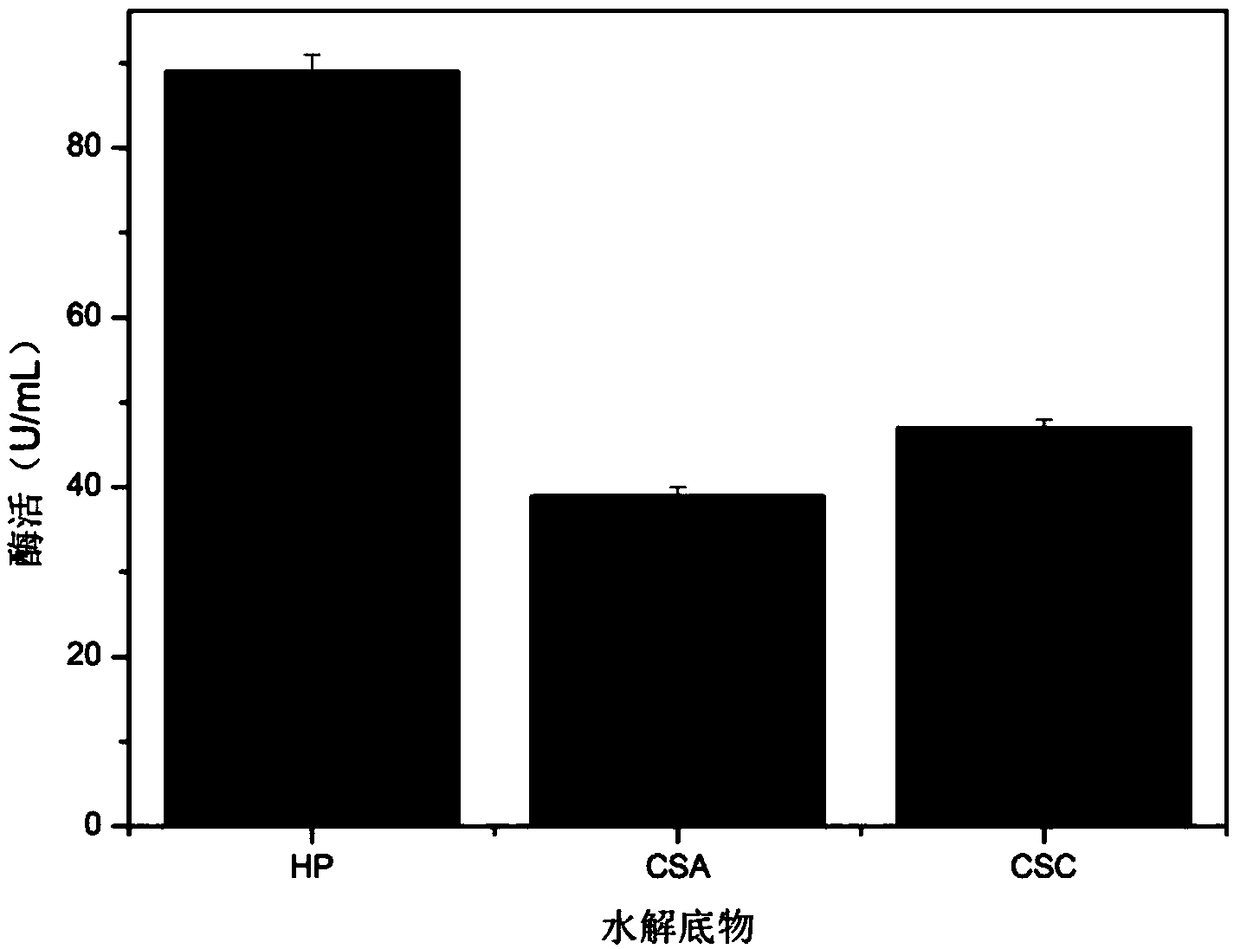
[0036]The recombinant Escherichia coli BL21DE3 / PET20-BpHEP containing the recombinant heparin hydrolase gene was used as the production strain, and a single colony was inoculated in LB medium, and cultivated overnight at 37°C and 220rmp to obtain the seed culture solution; Inoculate 1% of the inoculum into 25mL of TB medium, wait until OD 600 When it grows to 0.6, add 0.1MIPTG to the medium, induce the expression at 28°C, and induce expression for 28h. After the fermentation, the heparinase activity was determined to be 10 U / mL.
[0037] The recombinant Pichia pastoris GS115 / pPIC9K-BpHEP containing the recombinant heparin hydrolase gene was used as the production strain, and a single colony was inoculated in YPD medium, and cultured overnight at 28°C and 200rmp to obtain the seed culture solution; the seed culture solution Inoculate 25 mL of BMGY medium with an inoculation amount of 2% by m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com