Fluorine modified boron carbonitride photocatalytic material and application thereof to efficient reduction of carbon dioxide
A photocatalytic material, technology of fluorine-modified boron, applied in the application field of fluorine-modified boron-carbon-nitrogen photocatalyst material, efficient reduction of carbon dioxide, can solve the problems of inefficiency, environmental pollution, high cost, etc., and achieve low cost , mechanical wear resistance, high efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
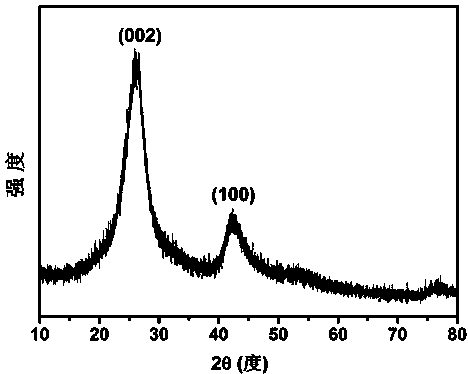
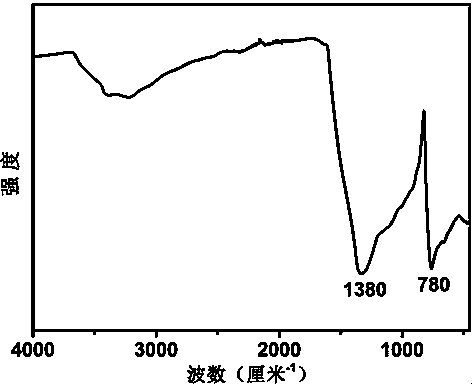
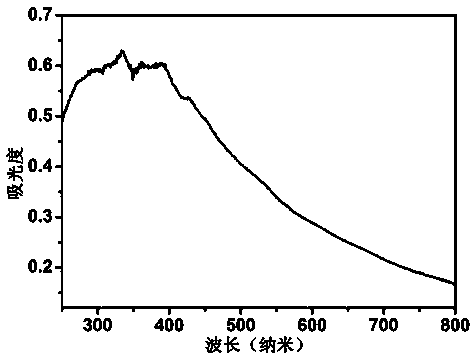
[0025] Completely dissolve 2g of boron oxide, 4g of urea, and 0.6g of glucose in 40-50ml of deionized water. After evaporating all the water at 75°C under normal pressure, place the resulting mixture in a corundum porcelain boat, and then place it in In a horizontal high-temperature tube furnace, the temperature was raised to 1250 °C at a rate of 5 °C / min in an ammonia atmosphere, and then kept for 5 h. After the sample was taken out, it was washed with 0.1 mol / L dilute hydrochloric acid, centrifuged, and dried to obtain Graphite phase boron carbon nitrogen powder; mix potassium fluoride with the obtained boron carbon nitrogen powder at a mass ratio of 0.4:1, grind them evenly, place them in a muffle furnace, and heat up to 400°C at a rate of 5°C / min in an air atmosphere , and then keep it warm for 3 h; after cooling to room temperature, take out the sample, wash it thoroughly with deionized water, filter it with suction, and dry it to obtain the fluorine-modified boron carbon ...
Embodiment 2
[0034] Completely dissolve 2g of boron oxide, 4g of urea, and 0.6g of glucose in 40-50ml of deionized water. After evaporating all the water at 75°C under normal pressure, place the resulting mixture in a corundum porcelain boat, and then place it in In a horizontal high-temperature tube furnace, the temperature was raised to 1250 °C at a rate of 5 °C / min in an ammonia atmosphere, and then kept for 5 h. After the sample was taken out, it was washed with 0.1 mol / L dilute hydrochloric acid, centrifuged, and dried to obtain Graphite phase boron carbon nitrogen powder; mix potassium fluoride with the obtained boron carbon nitrogen powder at a mass ratio of 0.2:1, grind them evenly, place them in a muffle furnace, and heat up to 400°C at a rate of 5°C / min in an air atmosphere , and then keep it warm for 3 h; after cooling to room temperature, take out the sample, wash it thoroughly with deionized water, filter it with suction, and dry it to obtain the fluorine-modified boron carbon ...
Embodiment 3
[0036] Completely dissolve 2g of boron oxide, 4g of urea, and 0.6g of glucose in 40-50ml of deionized water. After evaporating all the water at 75°C under normal pressure, place the resulting mixture in a corundum porcelain boat, and then place it in In a horizontal high-temperature tube furnace, the temperature was raised to 1250 °C at a rate of 5 °C / min in an ammonia atmosphere, and then kept for 5 h. After the sample was taken out, it was washed with 0.1 mol / L dilute hydrochloric acid, centrifuged, and dried to obtain Graphite phase boron carbon nitrogen powder; mix potassium fluoride with the obtained boron carbon nitrogen powder at a mass ratio of 0.3:1, grind them evenly, place them in a muffle furnace, and heat up to 400°C at a rate of 5°C / min in an air atmosphere , and then keep it warm for 3 h; after cooling to room temperature, take out the sample, wash it thoroughly with deionized water, filter it with suction, and dry it to obtain the fluorine-modified boron carbon ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com