Synthesis and application of fluorescent probe with specific recognition on GC33-3-1 antibody
A fluorescent probe and specific technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problems of affecting drug clearance rate, affecting the apparent distribution volume of drugs, etc., achieving simple synthesis method, simple post-processing, The effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of small molecule fluorescent probes for antibody labeling
[0038] (1) Synthesis of intermediate compound F1:
[0039]
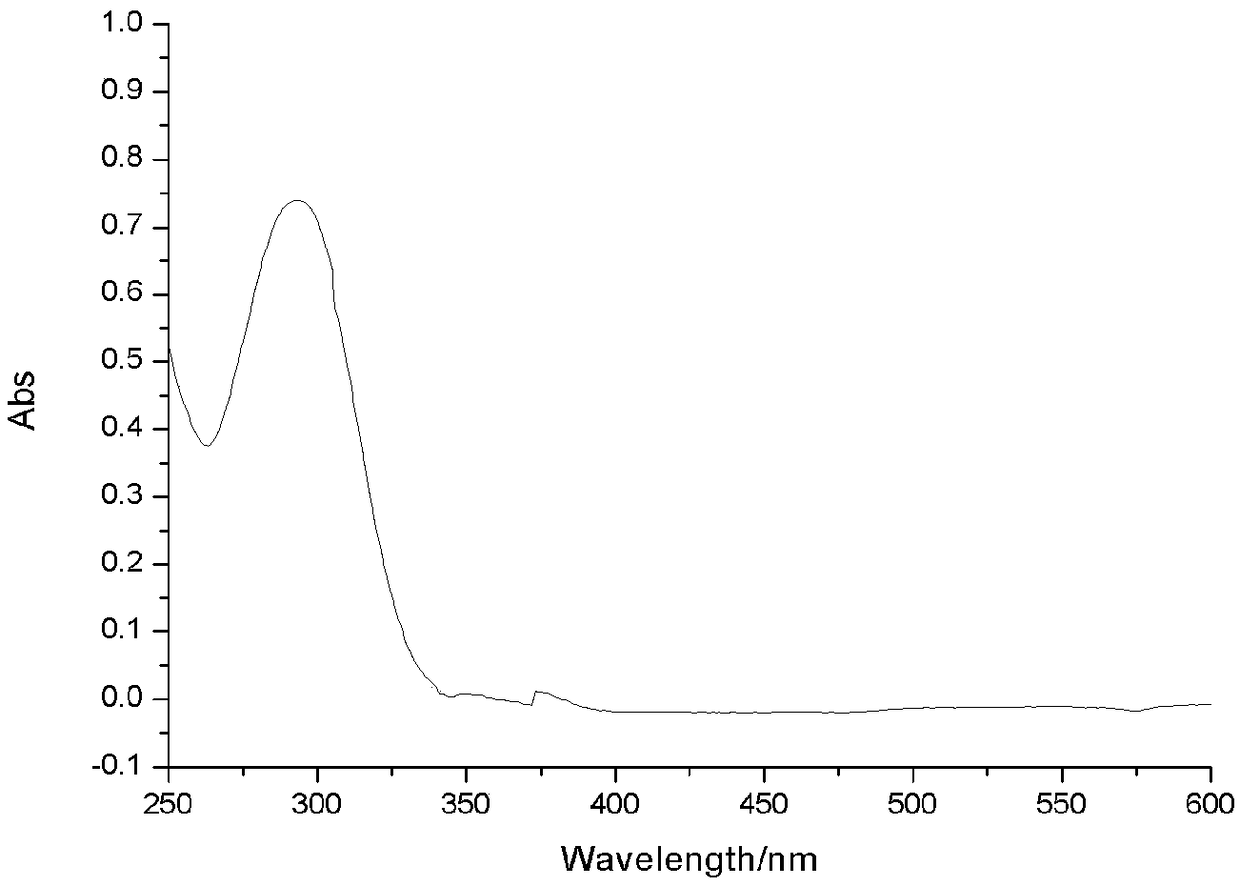
[0040] Weigh 15.7 mmol of 4,6-dichloropyrimidine and o-phenylenediamine, and 17.5 mmol of N,N-diisopropylethylamine in a microwave reaction tube, add 10 ml of isopropanol to dissolve, and react in the microwave at 150°C for 10 min. Post-processing: TLC was used to monitor the reaction results, and the reaction solution was evaporated under reduced pressure to remove the solvent to obtain yellow oil F1 with a yield of 100%, MS (m / z): 220.5.
[0041] (2) Synthesis of intermediate compound F2:
[0042]
[0043] Weigh 1.6 mmol of F1, 4.4 mmol of carbonyl-2--imidazole, and 4.4 mmol of pyridine into a round bottom flask, add tetrahydrofuran as a solvent, protect with nitrogen, and reflux in an oil bath at 65°C overnight. Post-processing: TLC was used to monitor the reaction results, the reaction solution was evaporated under r...
Embodiment 2
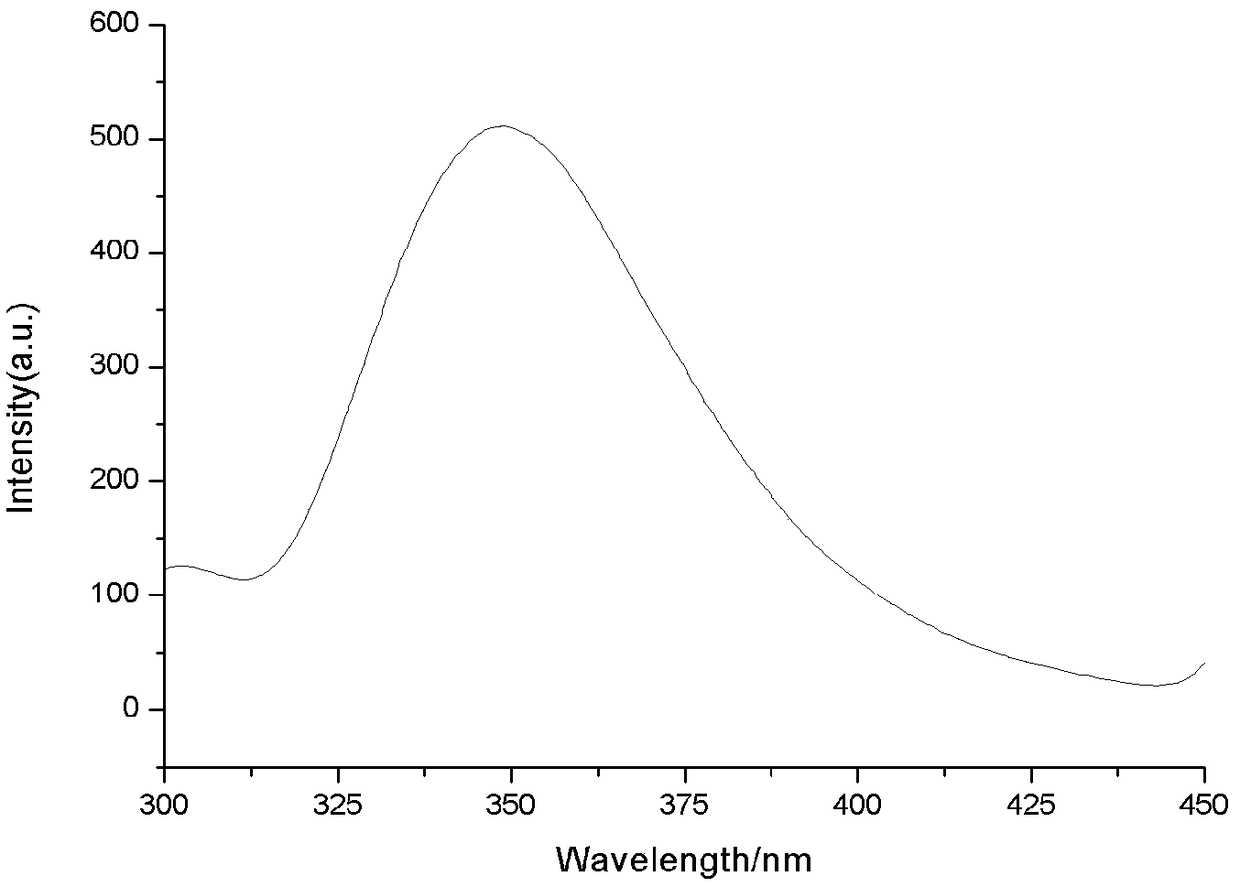
[0047] Example 2: Fluorescence changes after fluorescent probe-labeled human serum albumin (HSA) reaction
[0048] Sample solution: a certain amount of fluorescent probe prepared in Example 1 was dissolved in an appropriate amount of methanol solution and diluted with ultrapure water to a concentration of 1.5×10 -5 mol / L; Dilute human serum albumin (HSA) with ultrapure water to a concentration of 1X10 -5 mol / L; Tris-HCl solution is prepared to be 0.05mol / LPH=7.4 (contains 0.1mol / L NaCl); add 1ml Tris-HCl solution, 1ml fluorescent compound and 1 ml HSA solution, mix well. Control solution: 1 ml fluorescent probe solution, 1 ml Tris-HCl solution, and 1 ml ultrapure water were mixed to prepare a control solution. Determination of fluorescence spectrum image 3 .
Embodiment 3
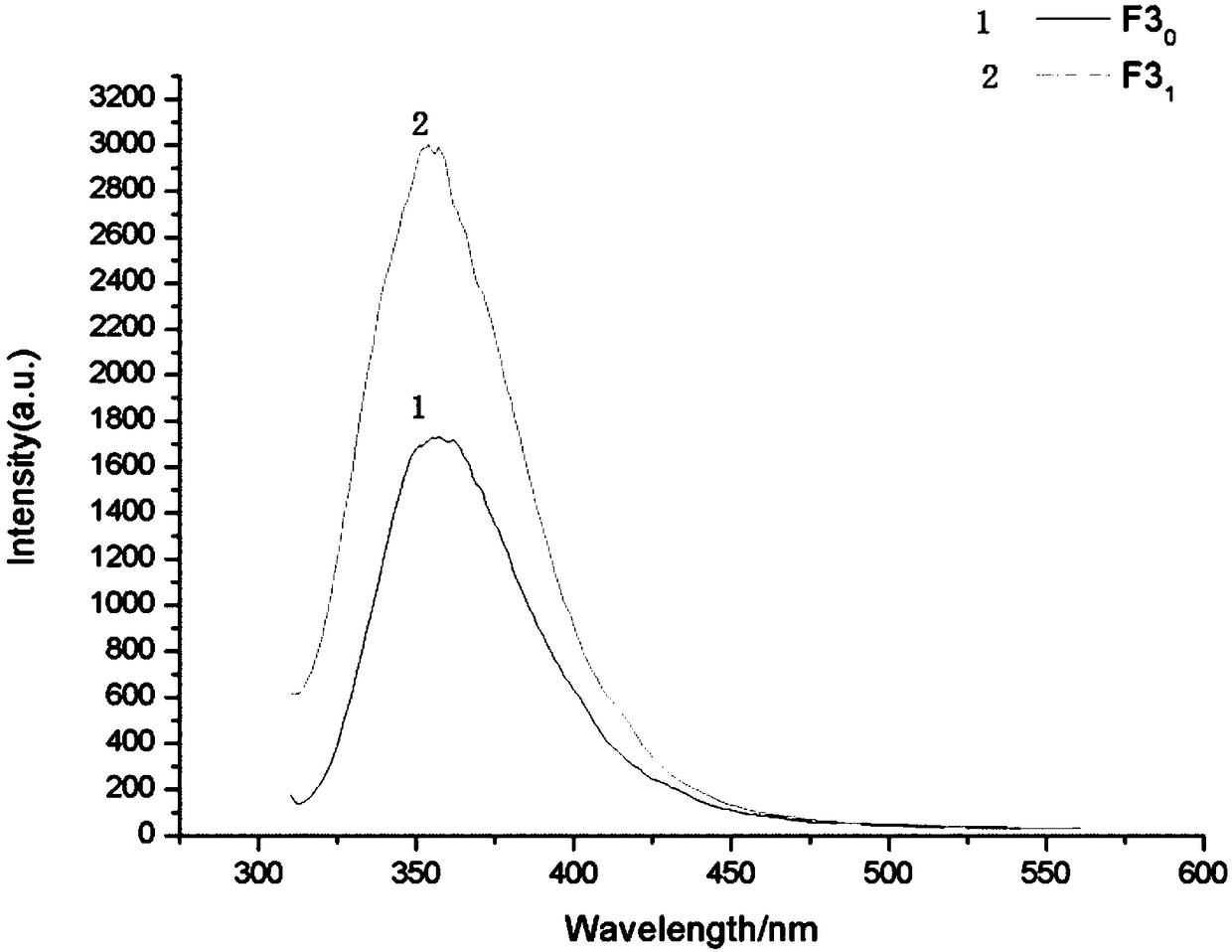
[0049]Example 3: Fluorescent probes directly recognize antibodies
[0050] (1) Coating: Dilute the antibody to 1 µg / ml, mix it with the coating solution (carbonate buffer) and add it to the enzyme-labeled plate, incubate at 37°C for 2 hours, and overnight at 4°C;
[0051] (2) Washing: Take 40 ml PBS and add 20 µl Tween 20 to make PBST solution, prepare PBST solution, wash the enzyme-labeled plate with PBST solution, 300 µl per well, 3 min each time, wash 3 times;
[0052] (3) Blocking: Bovine serum albumin was prepared to 0.02 mg / ml, added to the microtiter plate, and incubated at 37°C for 2 h;
[0053] (4) Washing: wash the enzyme-labeled plate with PBST solution, 300 µl per well, 3 min each time, and wash 3 times;
[0054] (5) Binding: Dissolve a certain amount of the fluorescent probe compound prepared in Example 1 in an appropriate amount of methanol solution and dilute it with ultrapure water to a concentration of 1.5X10 -5 mol / L; Tris-HCl solution was prepared to be 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com