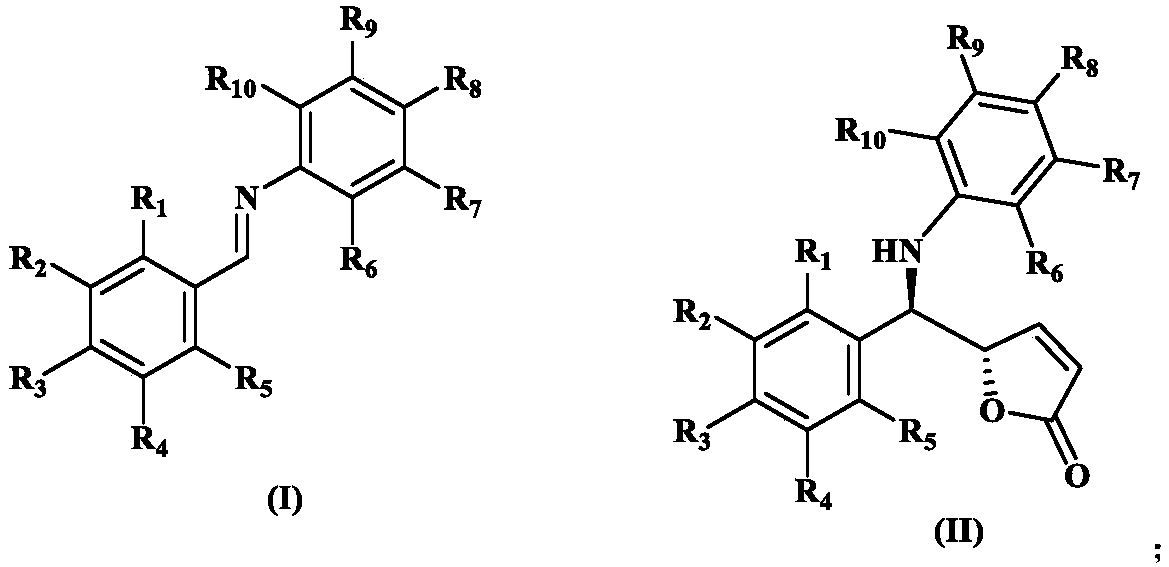
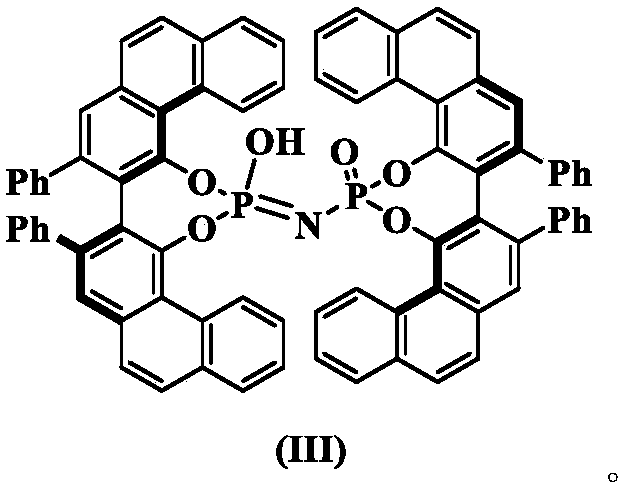
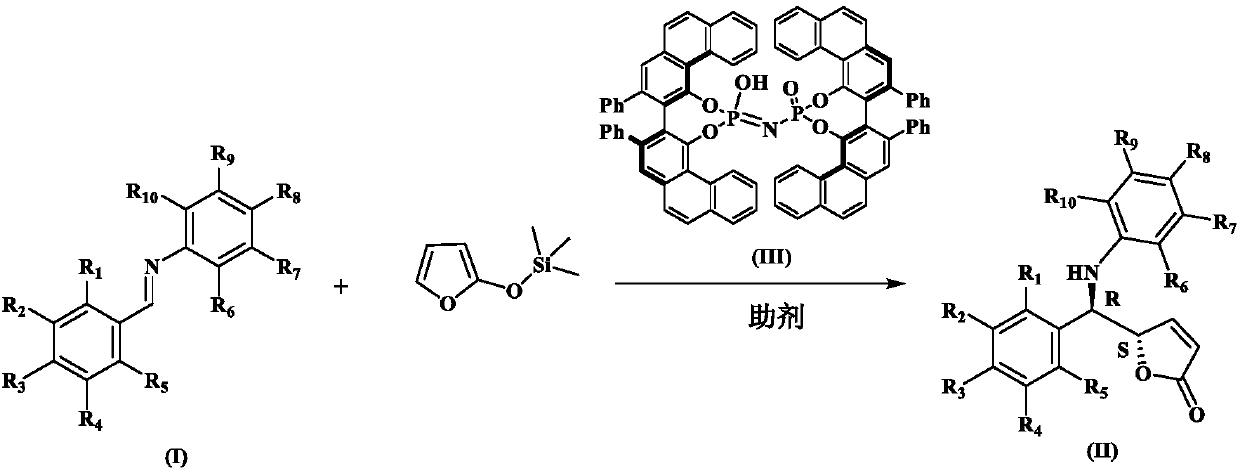
Synthesis method of chiral gamma-amine methylene-gamma-butylene lactone compound
A technology of aminomethylene and butenolactone, which is applied in the field of organic synthesis, can solve the problems of unfavorable development of new drugs and other products, complex raw material structure, heavy metal residues, etc., and achieve a wide range of substrates, simple steps, and no heavy metal residues Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Synthesis of (S)-5-((R)-phenyl(phenylamino)methyl)furan-2(5H)-one
[0025]
[0026] Dissolve 18g (0.1mol) of benzaldehyde aniline, 6g (5mmol) of (R,R)-phosphorimidic acid and 4g of hydroxypropyl-β-cyclodextrin in 1L of anhydrous toluene, stir for 15min, and cool down to -40°C, add 48 g (0.3 mol) of 2-(trimethylsilyloxy) furan under nitrogen protection, react for 10 hours and then perform silica gel column chromatography (eluent ethyl acetate:petroleum ether volume ratio=1:2) The product was purified to obtain 25.7 g of a colorless oily liquid with a yield of 97%, 80% ee, 93:7dr. Chiral OJ-H column, n-hexane:ethanol=80:20, flow rate 1.0mL / min, wavelength λ=254nm, retention time: t(primary)=27.865min, t(secondary)=32.746min; 1 H NMR (400MHz, DMSO-d 6 )δ7.81(d, J=8.0Hz,1H), 7.43(d,J=4.0Hz,2H), 7.30(t,J=8.0Hz,2H), 7.24–7.21(m,1H), 7.02( t,J=8.0Hz,2H), 6.68(d,J=8.0Hz,2H), 6.53(t,J=8.0Hz,1H), 6.40(d,J=8.0Hz,1H), 6.17–6.15( m,1H), 5.50(d,J=4.0Hz,1H), 4.90–4.8...
Embodiment 2
[0027] Example 2: Synthesis of (S)-5-((R)-phenyl(phenylamino)methyl)furan-2(5H)-one
[0028] Dissolve 18g (0.1mol) of benzaldehyde aniline, 6.7g (8mmol) of (R,R)-phosphorimidic acid and 4g of hydroxypropyl-β-cyclodextrin in 1L of anhydrous toluene, and stir for 15 minutes. Cool down to -40°C, add 48 g (0.3 mol) of 2-(trimethylsiloxy)furan under nitrogen protection, react for 10 hours and then perform silica gel column chromatography (eluent ethyl acetate:petroleum ether volume ratio=1: 2) The product was purified to obtain a colorless oily liquid (23.1 g, 91% yield, 72%ee, 91:9dr). The product structure is the same as in Example 1.
Embodiment 3
[0029] Example 3: Synthesis of (S)-5-((R)-phenyl(phenylamino)methyl)furan-2(5H)-one
[0030] Dissolve 18g (0.1mol) of benzaldehyde aniline, 3g (2.5mmol) of (R,R)-phosphorimidic acid and 4g of hydroxypropyl-β-cyclodextrin in 1L of anhydrous toluene, and stir for 15 minutes. Cool down to -40°C, add 48 g (0.3 mol) of 2-(trimethylsiloxy)furan under nitrogen protection, react for 10 hours and then perform silica gel column chromatography (eluent ethyl acetate:petroleum ether volume ratio=1: 2) The product was purified to obtain a colorless oily liquid (23.6 g, yield 92%, 75% ee, 89:11dr). The product structure is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com