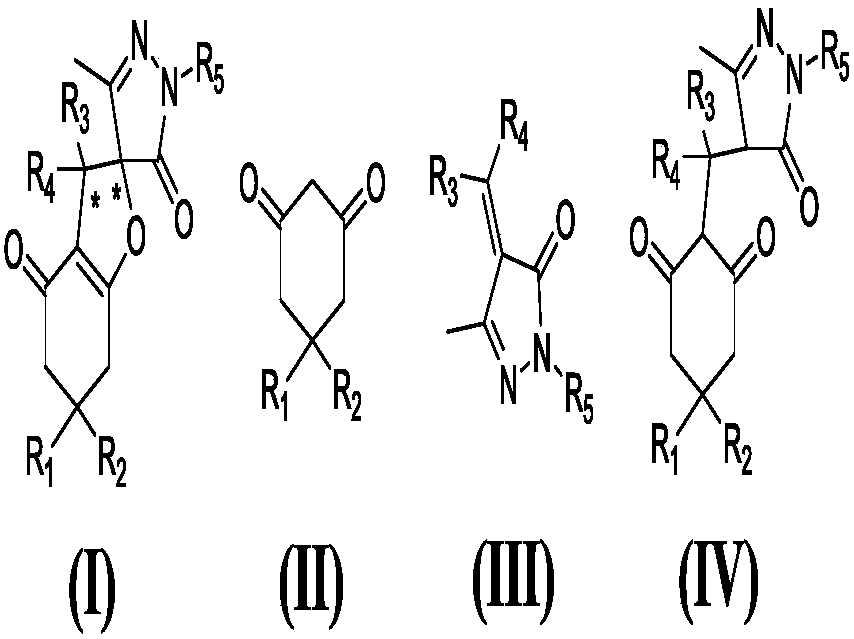
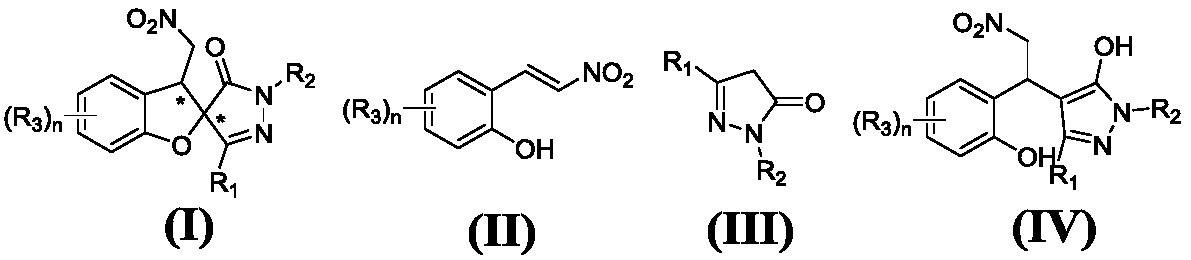
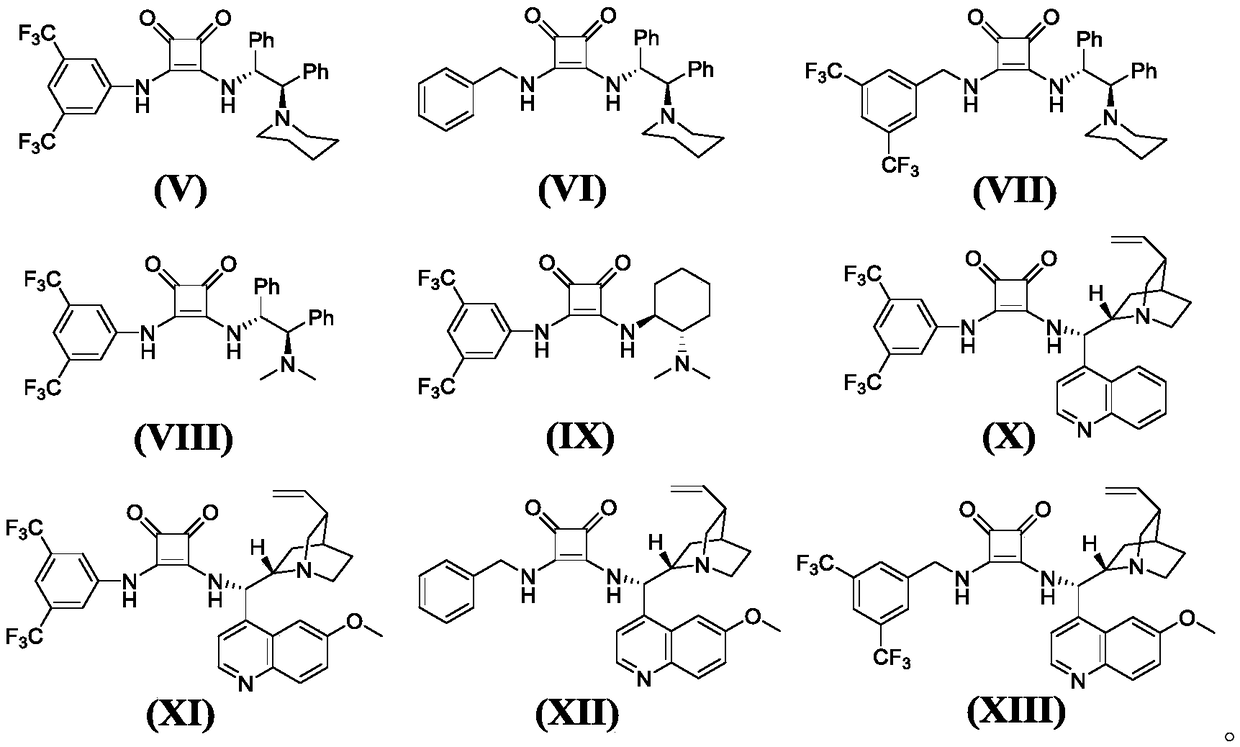
Series catalytic preparation method of chiral pyrazospirofurin compound
A technology for the preparation of pyrazole spirofuran and catalysis, which is applied in the direction of organic chemistry methods and organic chemistry, and can solve problems such as complex processes and excessive amounts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: (2S,3R)-3'-methyl-3-nitromethyl-1'-phenyl-3H-spiro[benzofuran-2,4'-pyrazole]-5'(1 'H)-ketone;
[0091]
[0092] (A) Take 10mL clean small test tube, add o-hydroxynitroalkene (0.2mmol, 0.033g), 5-methyl-2-phenyl-pyrazolone (0.2mmol, 0.0348g), chiral squaraine catalyst V (0.002mmol, 0.0012g), solvent dichloromethane (1mL), after reacting at 25°C for 6h, a mixture containing intermediate compound 1-A was obtained;
[0093] (B) Remove the solvent from the mixture containing intermediate compound 1-A, add cuprous iodide (0.076g, 0.4mmol), 30% hydrogen peroxide solution (0.4mmol, 0.0453g), solvent dichloromethane (1mL), After reacting at 25°C for 8h, extract with ethyl acetate (3×10mL), and desolvate the organic phase under reduced pressure, use ethyl acetate:petroleum ether=1:10 mixed solvent as eluent; 200-300 mesh column chromatography silica gel As filler, the target product (0.0593g, 88%yield, 92%ee,>99:1dr) obtained by column chromatography separation an...
Embodiment 2
[0094] Example 2: (2S,3R)-3'-ethyl-3-nitromethyl-1'-phenyl-3H-spiro[benzofuran-2,4'-pyrazole]-5'(1 'H)-ketone;
[0095]
[0096] (A) Take a 10mL clean small test tube, add o-hydroxynitroolefin (0.2mmol, 0.033g), 5-ethyl-2-phenyl-pyrazolone (0.2mmol, 0.0376g), chiral squaraine catalyst VI (0.02mmol, 0.0093g), solvent toluene (2mL), after reaction at 25°C for 48h, a mixture containing intermediate compound 2-A was obtained;
[0097] (B) The mixture containing intermediate compound 2-A was desolvated, then added iodobenzene acetate (0.8mmol, 0.2576g), 70% hydroperoxide tert-butanol aqueous solution (0.2mmol, 0.0257g), solvent toluene ( 2mL), after reacting at 25°C for 48h, extracted with ethyl acetate (3×10mL), and the organic phase was precipitated under reduced pressure, using ethyl acetate:petroleum ether=1:10 mixed solvent as eluent; 200-300 mesh column Chromatographic silica gel is used as filler, and the target product (0.0604g, 86%yield, 92%ee,>99:1dr) obtained by col...
Embodiment 3
[0098] Example 3: (2S,3R)-3-nitromethyl-1'-phenyl-3'-propyl-3H-spiro[benzofuran-2,4'-pyrazole]-5'(1 'H)-ketone;
[0099]
[0100] (A) Take 10mL clean small test tube, add o-hydroxynitroalkene (0.2mmol, 0.033g), 5-propyl-2-phenyl-pyrazolone (2mmol, 0.404g), chiral squaraine catalyst VII (0.01mmol, 0.0060g), solvent acetonitrile (4mL), after reacting at 40°C for 1h, a mixture containing intermediate compound 3-A was obtained;
[0101] (B) Remove the solvent from the mixture containing intermediate compound 3-A, then add tetrabutylammonium iodide (0.5mmol, 0.1845g), 85% m-chloroperbenzoic acid solution (2mmol, 0.407g), solvent Acetonitrile (4mL), after reacting at 40°C for 2h, extracted with ethyl acetate (3×10mL), the organic phase was precipitated under reduced pressure, using ethyl acetate:petroleum ether=1:10 mixed solvent as eluent; 200-300 The column chromatography silica gel is used as filler, and the target product (0.0593g, 81%yield, 92%ee,>99:1dr) obtained by colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com