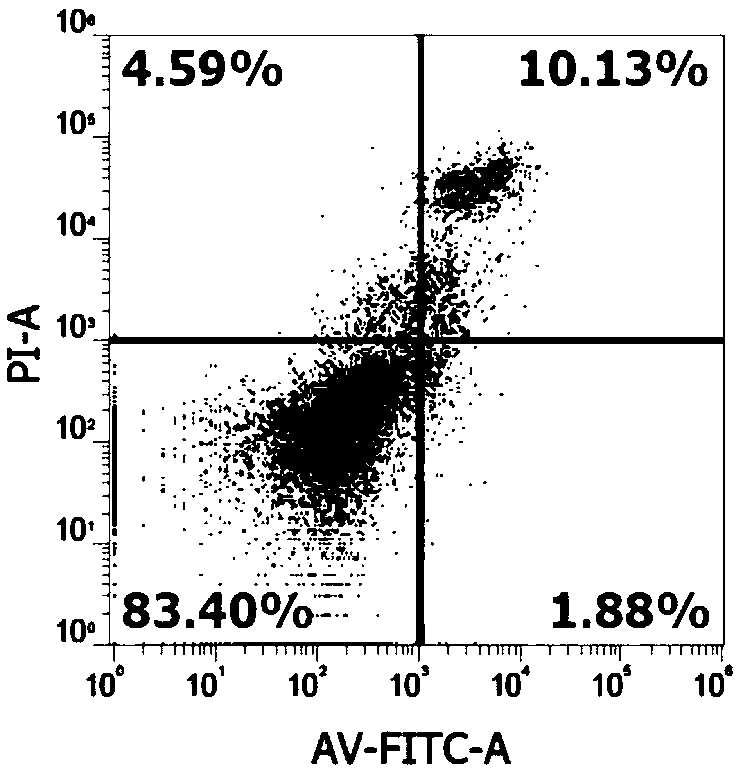
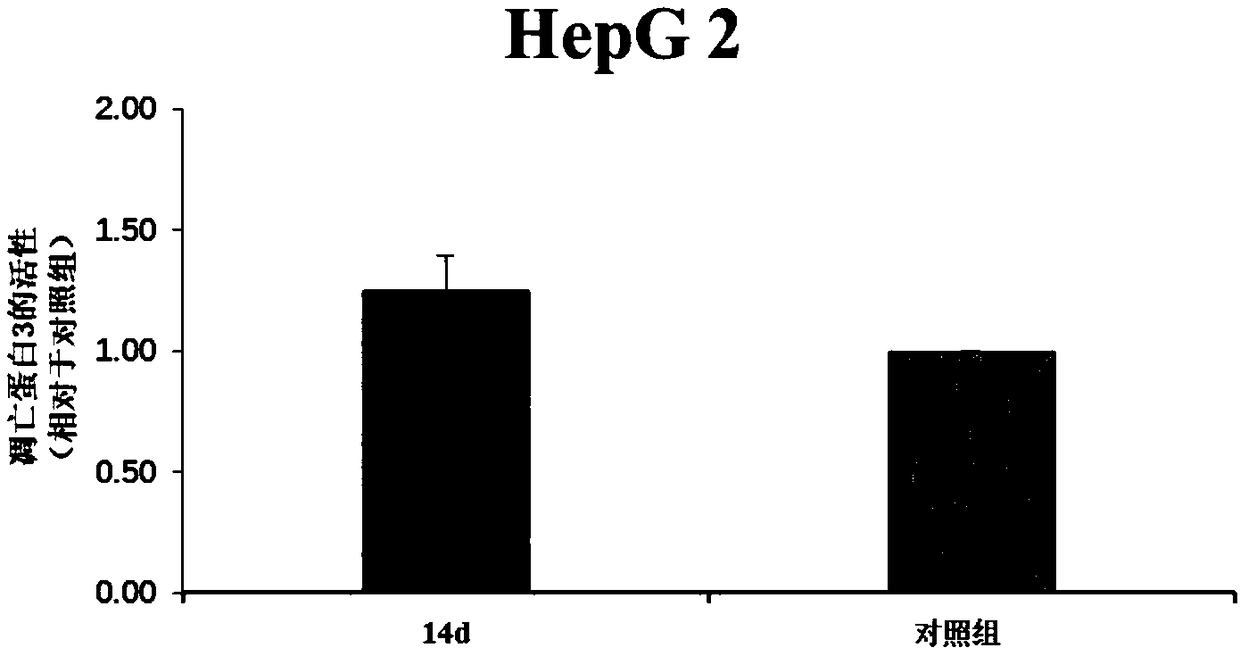
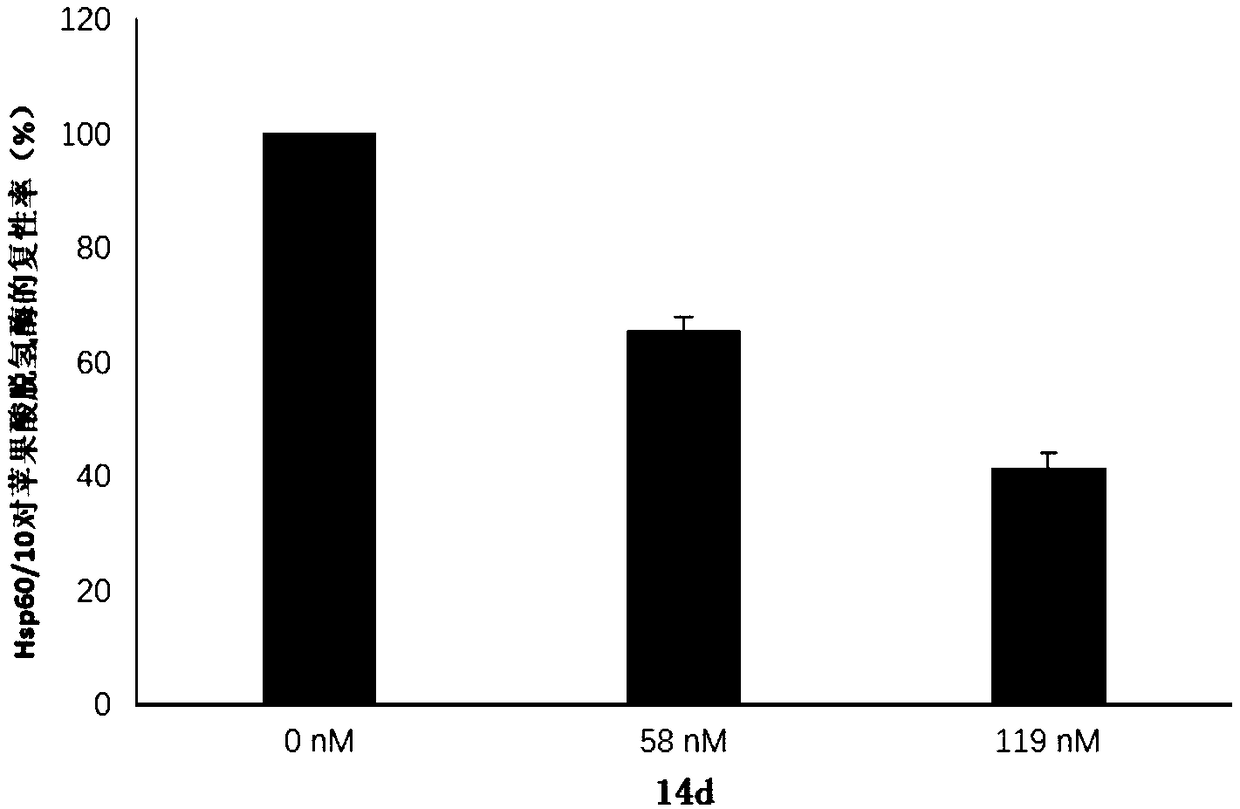
Flavone alkoxy phosphoramidate derivative for targeted Hsp60, preparation and application
A technology for flavonoid alkoxy phosphoramidate and derivatives, which is applied in the field of pharmaceutical compounds, can solve problems such as poor solubility, difficult absorption and metabolism, and achieves the effects of low cost, good antitumor activity, good practical value and application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 chrysin-7-ethoxyglycine phosphoramidate
Embodiment 1-1
[0030] Example 1-1: Phenyl dichlorophosphate (1.5mL, 10mmol, 0.4M) was added to a 250mL three-necked flask, then dichloromethane (100mL) was added to dissolve it, and the reaction system was cooled to At -83.5°C, TEA (3 mL, 10 mmol, 1 equiv) was added dropwise to the reaction solution using a constant pressure dropping funnel for 10 min. Then, a mixed solution of 2-bromoethanol (1.1 mL, 8 mmol, 0.8 equiv) and dichloromethane (5 mL) was added dropwise to the above reaction solution, and the dropwise reaction was completed at low temperature for 1 h. Naturally rise to room temperature to react. The progress of the reaction was detected by TLC, and the developer was chloroform:methanol=30:1. The reaction time is about 12h. Add anhydrous dichloromethane (50mL), weigh glycine methyl ester hydrochloride (1.26g, 10mmol, 1equiv) and add to the above reaction system, add TEA (1.5mL) dropwise to the reaction solution with a constant pressure dropping funnel , 20mmol, 2equiv), added d...
Embodiment 1-2
[0031] Example 1-2: Add chrysin (0.254g, 1mmol, 0.01M) in a 250mL three-necked flask, then add acetone (100mL) to dissolve it, add cesium carbonate (0.488g, 1.5mmol, 1.5equiv), and then add Ethoxyglycine phosphoramidate (1.840g, 5mmol, 5equiv), the reaction was placed in an oil bath at 50°C for reaction, and the reaction progress was detected by TLC. The developing agent was petroleum ether: ethyl acetate=3:2, and the reaction time was 6~ 7d, after the reaction is over, stop stirring. Post-treatment: filter to remove insoluble matter, evaporate the solvent under reduced pressure to obtain a yellow oily liquid, which is separated by column chromatography. The column chromatography conditions are petroleum ether:ethyl acetate=3:2, and after concentration, a light yellow solid is obtained. The yield is 10%. The results of instrumental analysis of the compound structure: 1 H NMR (400MHz, DMSO-d6 ), δ: 12.82(s, 1H, 5-OH), 8.10(d, 2H, J=6.8Hz, H-2′, H-6′), 7.64(m, 1H, H-4′), 7.60 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com