Escherichia coli expression system containing manganese ion recombinant protein, and application method thereof
A technology of Escherichia coli and expression system, applied in microorganism-based methods, recombinant DNA technology, biochemical equipment and methods, etc., can solve the problems of complex renaturation process, high cost, inactivity, etc., and achieve simple separation and purification process, The effect of low production cost and increased vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1 Design of the enzyme protein gene expression cassette containing manganese ions
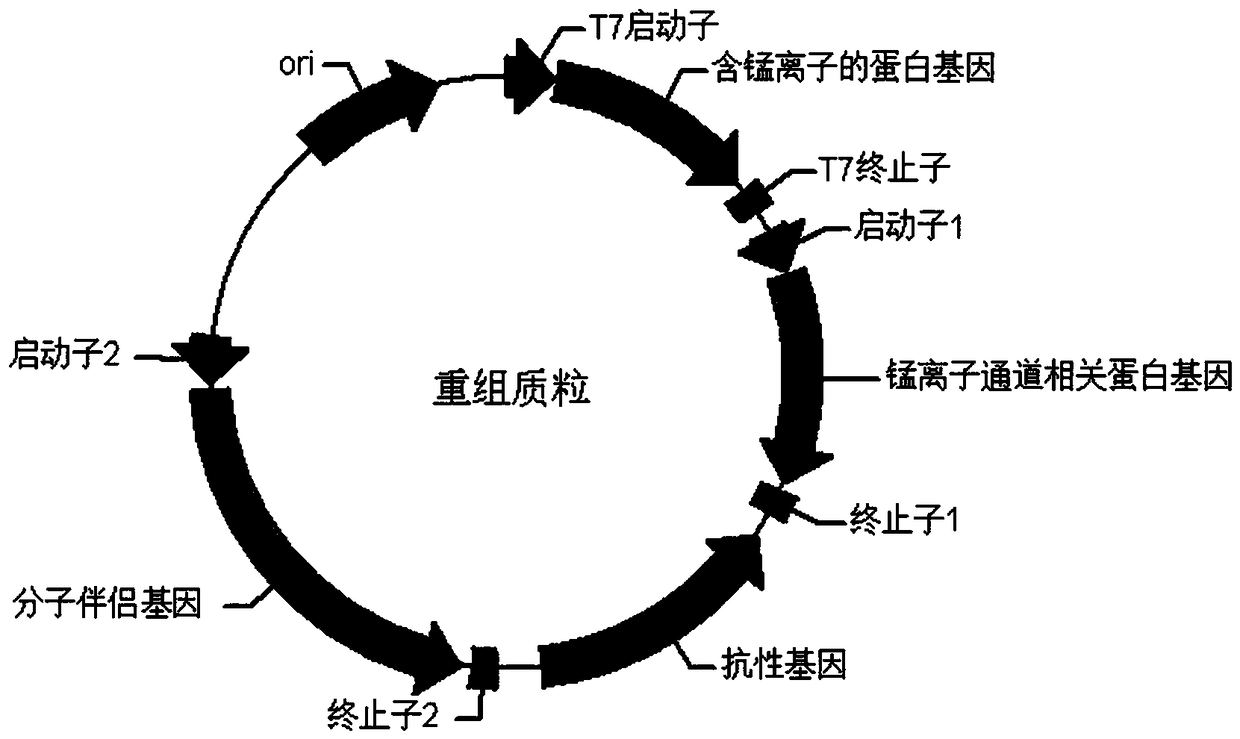
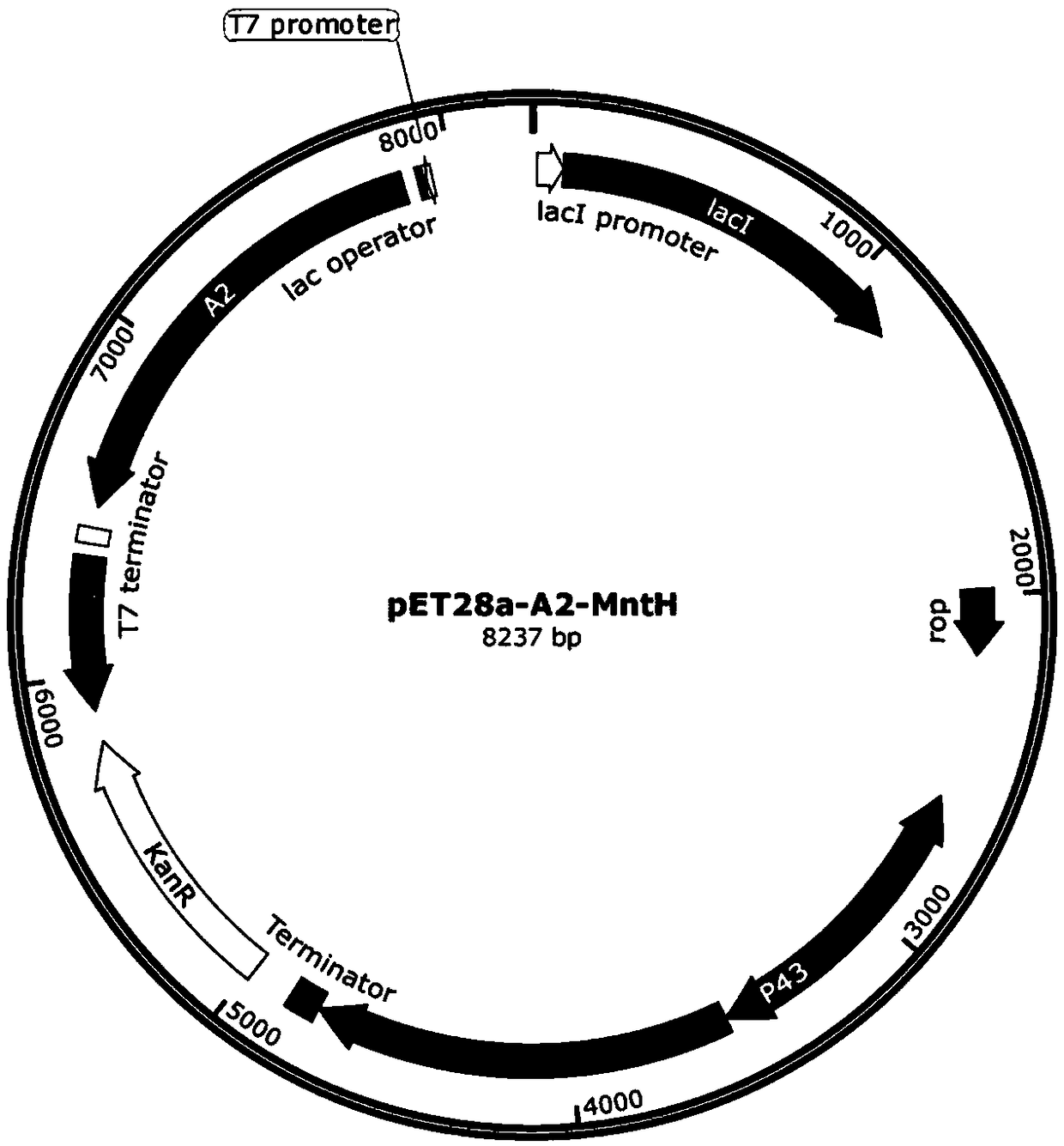
[0095] The schematic diagram of the universal vector for the co-expression of the Escherichia coli molecular chaperone gene, the enzyme protein gene containing manganese ions and the channel protein gene related to the pumping of manganese ions constructed in this example is as follows figure 1 shown. Among them, promoter 1 and promoter 2 are medium-strength promoters or weak promoters that can promote the expression of molecular chaperone genes and manganese ion channel-related protein genes in Escherichia coli, such as the P43 promoter sequence (SEQ ID NO.6, from subtilis), M1-93 promoter (SEQ ID NO. 7), araBAD promoter (SEQ ID NO. 8) and Lac promoter (SEQ ID NO. 9). Promoter 1 and promoter 2 can be the same promoter, or different promoters can be selected. The molecular chaperone gene is one or more of dnaK-dnaJ-grpE, groES-groEL, groES-groEL-tig or tig. The manganese io...
Embodiment 2
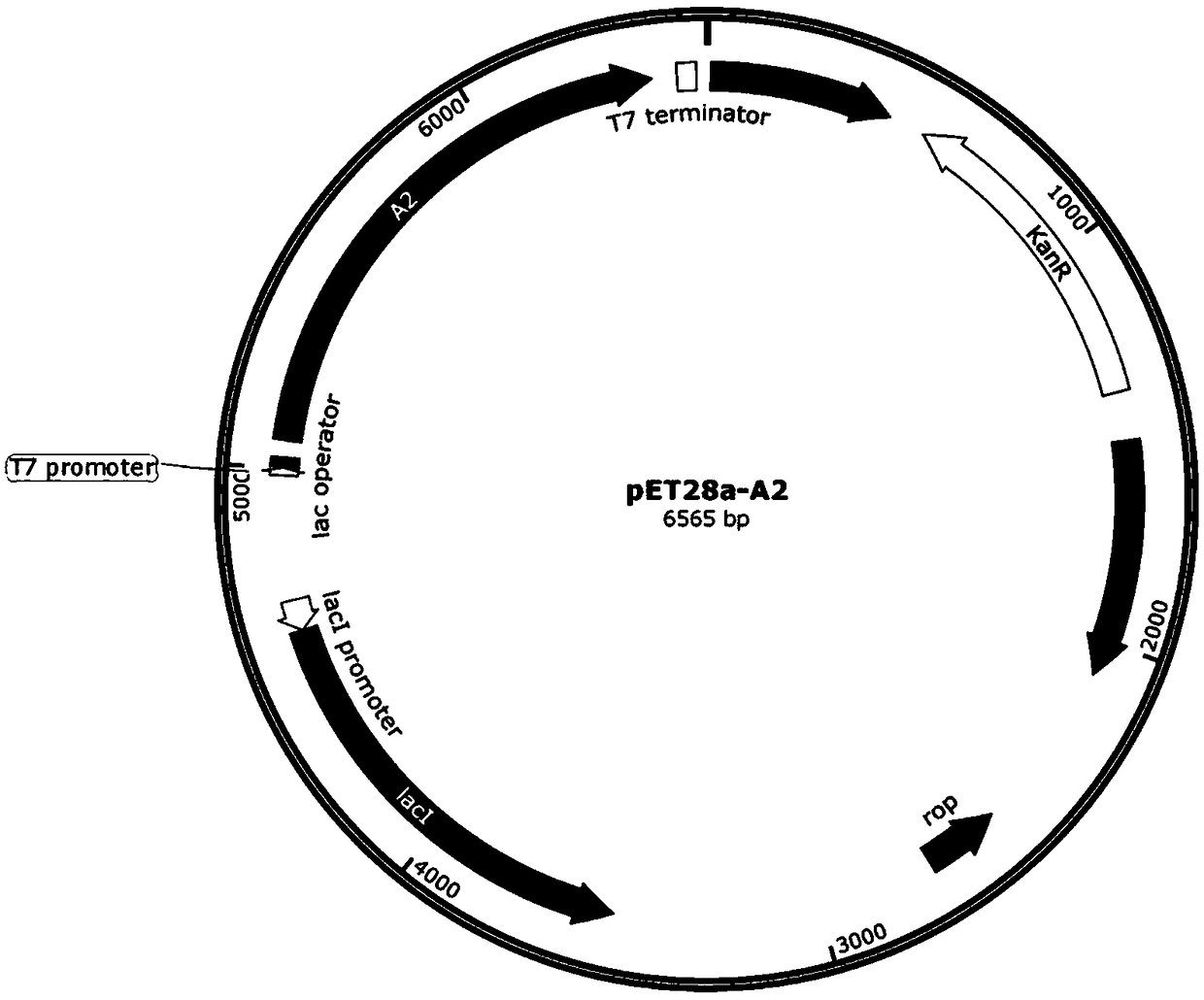
[0096] Example 2 Construction of oxalate decarboxylase A2 gene expression plasmid pET28a-A2
[0097] Using the A2 gene (SEQ ID NO.1) synthesized from the whole gene as a template, design a primer pair F1 / R1 to amplify the gene, and perform gel recovery and purification on the amplified product. The method refers to the commercially available DNA mini-purification kit. According to the method described in the specification, the DNA fragment 1 (ie, the A2 gene fragment) is finally obtained. The PCR system is: 10×PCR Buffer 5μL, 2mM dNTP 5μL, 25mMMgSO 45 μL, 1.5 μL each of 10 μM primer F / R, 0.5 μL template DNA, 1 μL KOD-Plus-Neo, 32.5 μL ddH2O; PCR reaction conditions are as follows: 94°C for 3 min, 30 cycles (98°C for 10s, 60°C for 30s, 68°C for 35s ), 68°C for 5 minutes, and 4°C for 10 minutes; the PCR system in the description of the following vector construction is consistent with the above description, and will not be repeated below. The PCR reaction conditions are slightly...
Embodiment 3
[0104] Example 3 Molecular chaperone overexpression strain construction
[0105] Transform five kinds of commercial chaperone plasmids pG-KJE8, pGro7, pKJE7, pG-Tf2 and pTf16 into commercial strains, screen positive clones on the LB solid medium plate containing 20 μg / ml, and use BL21(DE3) and Origami2 (DE3) expression strains were used as host bacteria to construct molecular chaperone overexpression strains. Competent cell preparation and E. coli transformation refer to "Molecular Cloning Experiment Guide (3rd Edition)" to obtain a series of strains: pG-KJE8 / BL21(DE3), pGro7 / BL21(DE3), pKJE7 / BL21(DE3), pG- Tf2 / BL21(DE3), pTf16 / BL21(DE3), pG-KJE8 / Origami2(DE3), pGro7 / Origami2(DE3), pKJE7 / Origami2(DE3), pG-Tf2 / Origami2(DE3) and pTf16 / Origami2( DE3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com