Applications of compound containing N-oxidized tertiary amine group as cell mitochondria targeting carrier
A technology for oxidizing tertiary amine groups and tertiary amines, applied in the field of medicine, can solve problems such as toxic and side effects, and achieve the effects of reducing toxic and side effects, good water solubility and biocompatibility, and reducing enrichment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
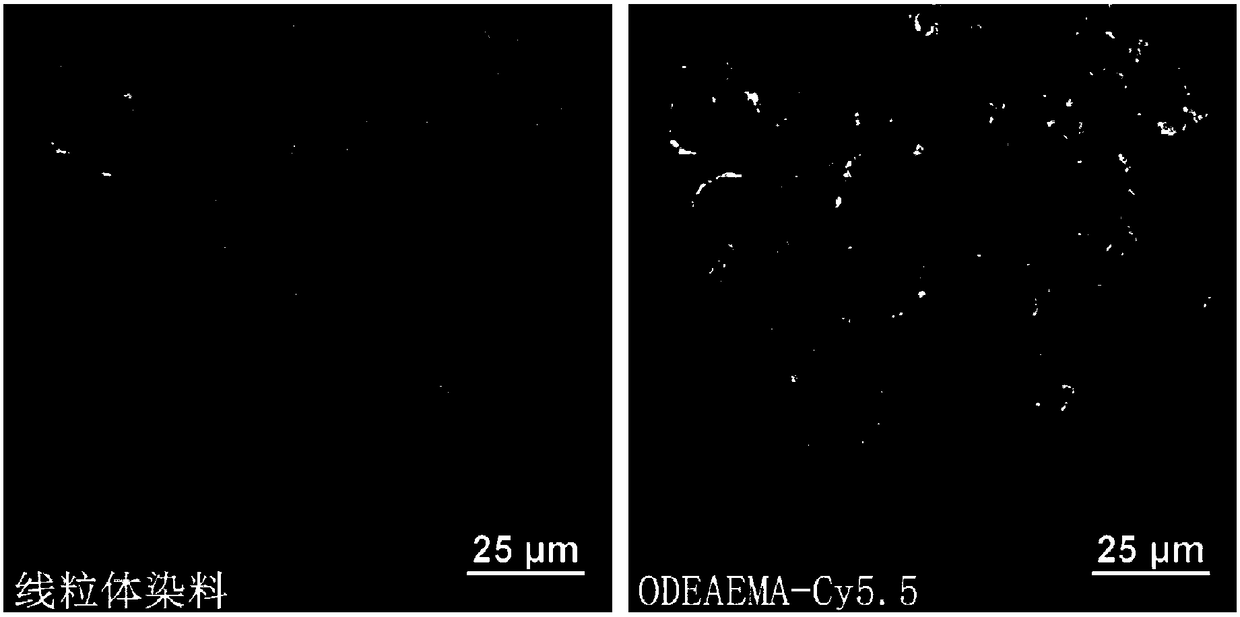
[0062] Embodiment 1: ODEAEMA-Cy5.5 fluorescent probe synthesis
[0063] The present application is based on the application of N-oxidized tertiary amine molecules as mitochondrial fluorescent probes, one embodiment of which is N-oxidized-methacrylic acid 2-(N,N-diethylamino)ethyl ester (ODEAEMA) Molecule-linked fluorescent molecules serve as mitochondrial fluorescent probes. More specifically, in one embodiment, the linked fluorescent molecule is Cy5.5, which is synthesized as follows:
[0064]
[0065] The synthesis method is as follows:
[0066] 2-(N,N-Diethylamino)ethyl methacrylate (DEAEMA, 9.25 g) was dissolved in 20 mL of chloroform. The reaction system was placed in an ice-water bath, and a mixed solution of 11.17 g m-chloroperoxybenzoic acid and 100 mL chloroform was slowly added dropwise to the system. After the reaction system was stirred at room temperature for 12 hours, 17 g of potassium carbonate was slowly added. After stirring for 10 minutes, the reaction...
Embodiment 2
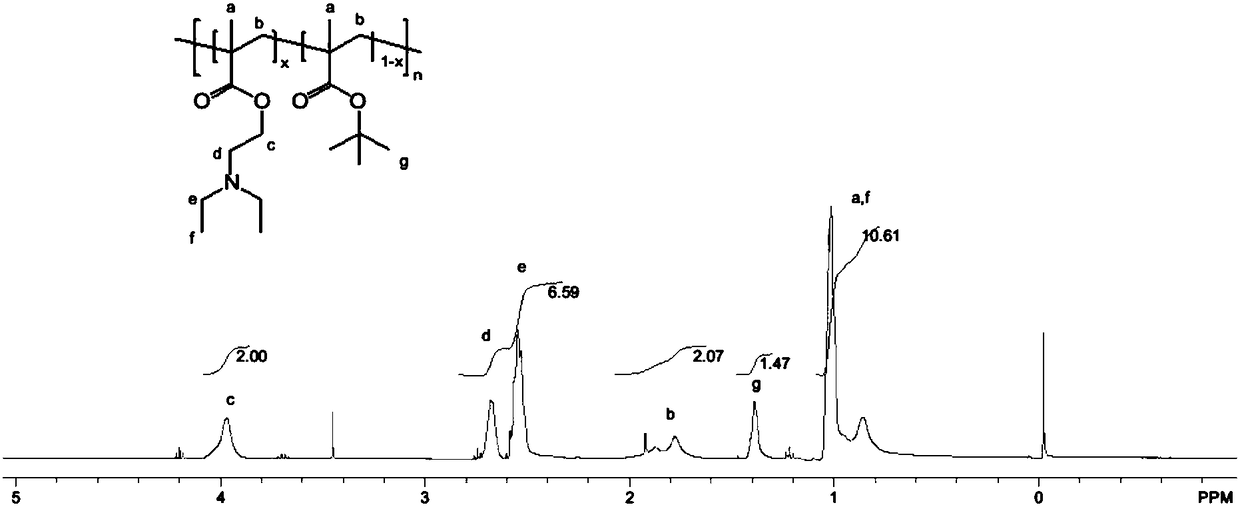
[0069] Example 2: PODEAEMA Targets Mitochondrial Carrier Synthesis
[0070] An embodiment of the application of the N-oxidized tertiary amine-based polymer as a mitochondria-targeting carrier includes linking fluorescent molecules to prepare a mitochondrial fluorescent probe-type polymer. More specifically, in one embodiment, the loaded fluorescent molecule is Cy5.5, which is synthesized as follows:
[0071]
[0072] Wherein the mole fraction x is 0.8-0.95, n=3-300.
[0073] The synthesis method is as follows:
[0074] (1) Synthesis of PODEAEMA-NHS.
[0075] 2-(N,N-diethylamino)ethyl methacrylate (DEAEMA, 5.37g) and tert-butyl methacrylate (tBMA, 0.37g), 0.0041g of ethyl 2-isobromobutyrate, After putting 0.085 g of 2,2'-bipyridine, 0.038 g of cuprous bromide and 2 mL of methanol into the polymerization bottle, nitrogen gas was continuously fed for 30 minutes to remove oxygen. Then seal and react at 30°C for 6 hours. After the reaction was finished, the catalyst was rem...
Embodiment 3
[0084] Embodiment 3: Preparation of PODEAEMA-b-polySN38 block copolymer
[0085] The application of the N-oxidized tertiary amine polymer as a drug carrier in the present application, one embodiment is to use the N-oxidized tertiary amine polymer as a hydrophilic chain, which is connected to the drug molecule or its polymer to form Amphiphilic, bonded drugs form nanoparticles or micelles by their assembly in aqueous solution. More specifically, in one embodiment, the loaded drug is 7-ethyl-10-hydroxycamptothecin (SN38), which is synthesized as follows:
[0086]
[0087] Wherein, m=1-200, n=1-300.
[0088] The synthesis method is as follows:
[0089] After putting DMAEMA (240mg), ethyl 2-isobromobutyrate (3.9mg), pentamethyldiethylenetriamine (40mg), cuprous bromide (10mg), and DMF1mL into the polymerization bottle, nitrogen gas was continuously introduced Oxygen was removed for 30 minutes. Then seal and react at 40°C for 4 hours. Under the protection of argon, directly i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com