Carbon nanotubes-supported nitrogen doped graphene-coated platinum nanometer compound material and preparation method and application thereof
A technology of nitrogen-doped graphene and carbon nanotubes, which is applied in chemical instruments and methods, hydrogenation to hydrocarbons, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low styrene selectivity and complicated preparation methods , low conversion rate and other problems, to achieve the effect of simple preparation method, low cost and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
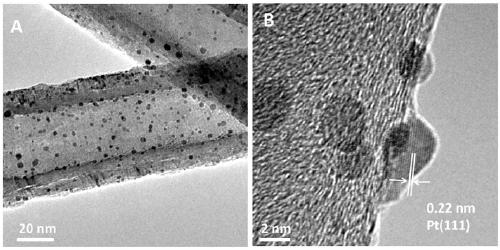
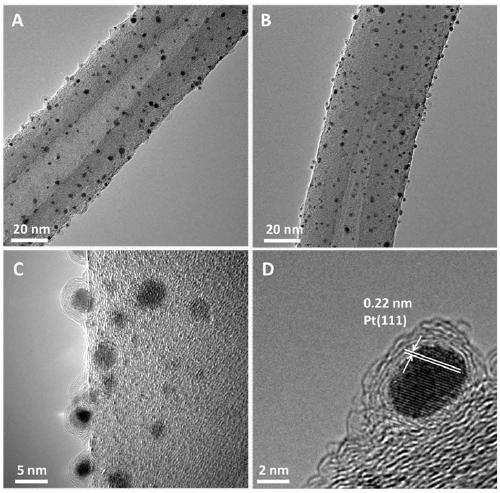
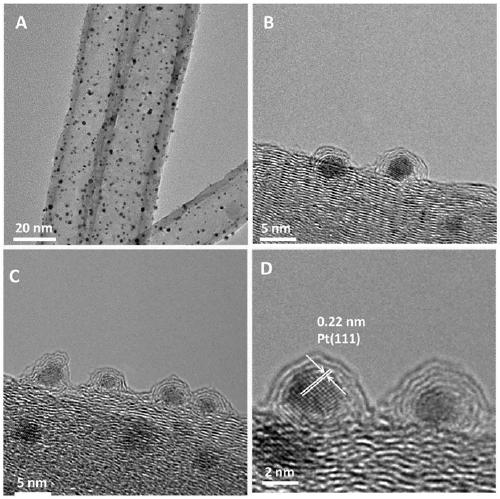
[0028] Example 1 CN@Pt / CNTs of carbon nanotube-supported nitrogen-doped graphene-coated platinum nanocomposites
[0029] (1) The preparation method is as follows:
[0030] 1. Preparation of Pt / CNTs
[0031] 1) Mix 1500 mg of carbon nanotubes and 150 mL of concentrated nitric acid, heat to reflux at 120° C. for 12 hours, filter, and wash until neutral to obtain acidified carbon nanotubes.
[0032] 2) Weigh 200 mg of acidified carbon nanotubes, put them into 40 mL of ethylene glycol solution, and sonicate for 3-10 minutes, so that the carbon nanotubes are evenly dispersed. Then add 1.325mL chloroplatinic acid solution, adjust the pH of the mixed solution to 11-14, react at a constant temperature of 130°C for 3h, cool to room temperature after the reaction, adjust the pH of the mixed solution to 3-5, wash with deionized water, and place Dry overnight in an oven at 60-70°C to obtain a platinum nanoparticle composite material supported by carbon nanotubes, which is denoted as Pt / ...
Embodiment 2
[0042] Example 2 Application of Pt / CNTs, C@Pt / CNTs and CN@Pt / CNTs
[0043] 1. Method: Add 10mg of Pt / CNTs, C@Pt / CNTs and CN@Pt / CNTs to 200μL of ethanol solution of phenylacetylene and n-octane (200μL of n-octane as internal standard dissolved in 10mL of ethanol), Then put them together into a high-pressure reactor, exchange the air in the reactor with 0.04MPa argon for several times, then raise the temperature to 50°C, feed in 0.3MPa hydrogen, and react at 50°C for 20min to 120min. The conversion rate of phenylacetylene and the selectivity of styrene were detected by gas chromatography.
[0044] Depend on Figure 4a It can be seen that, using Pt / CNTs as a catalyst, with the increase of reaction time, the conversion rate increases gradually, and after the conversion rate reaches 100%, the selectivity gradually decreases. When the reaction time is 100 minutes, the conversion rate of phenylacetylene is 100%, and the conversion rate of benzene The selectivity to ethylene was 17....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com