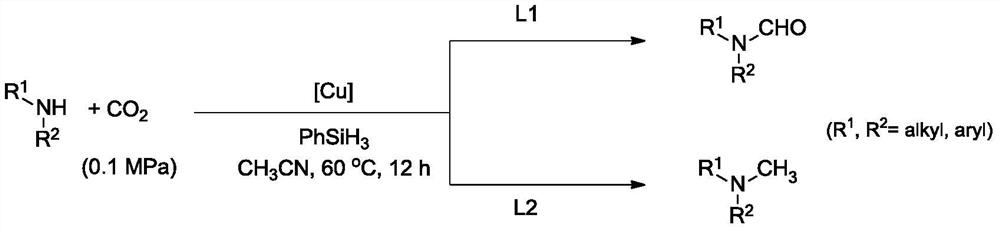
A method for preparing formamide and n-methylamines by selective reduction of carbon dioxide and amines regulated by ligands
A technology of carbon dioxide and methylamine, applied in chemical instruments and methods, preparation of reductive alkylation, preparation of carboxylic acid amide, etc., can solve the problems of complex factors affecting reaction conditions, changing temperature, etc., and achieve wide application range of substrates, Reduction of reaction cost, reduction of reaction risk factor and effect of reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The specific steps of the preparation method are:
[0034] Step 1 Add amines, hydrosilanes, catalysts, ligands and solvents into the reactor, wherein the molar ratio of catalysts to ligands is 1:2-1:4, and the molar ratio of catalysts to amines is 2.5%-5 %, the molar ratio of amines to hydrosilane is 1:2-3. After mixing, fill the reactor with 0.1-8.0MPa of CO 2 ;
[0035] Step 2: control the reaction temperature to 20-80°C, and react for 10-48h;
[0036] After the reaction in Step 3 is completed, the reaction system is cooled to room temperature and separated by column chromatography to obtain formamide and N-methylated products.
[0037] The raw material amines can be selected as secondary amines. Wherein the ligand can be o-phenanthroline, three (4-methoxyphenyl) phosphine, three (4-trifluoromethylphenyl) phosphine, three (4-methylphenyl) phosphine, three (4- Chlorophenyl)phosphine, tricyclohexylphosphine, diphenylcyclohexylphosphine, 1,4-bis(diphenylphosphine)bu...
Embodiment 1
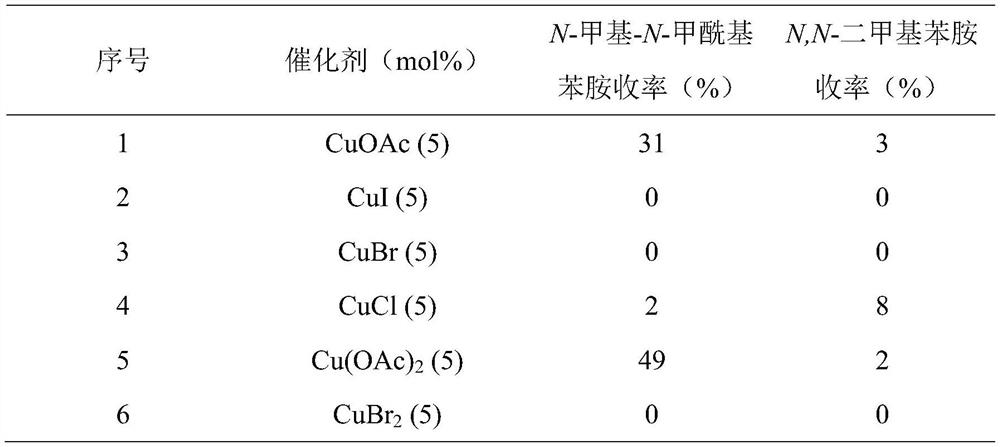
[0041] The influence of embodiment 1 catalyst on formamide and N-methylamines yield
[0042] A ligand-regulated CO 2 The method for preparing formamide and N-methylamines through selective reduction of amines, the steps are as follows:
[0043] 1) Add catalyst, 4.5mg (5mol%) o-phenanthroline, acetonitrile 2mL, 54 μL (0.5mmol) N-methylaniline, 0.2mL (1.5mmol) phenylsilane into a 10mL Schlenk tube, and then add connected to a CO filled 2 the balloon;
[0044] 2) The reaction temperature is controlled at 50°C, and the reaction time is 12 hours;
[0045] 3) After the reaction was completed and the reaction system was cooled to room temperature, 5 mL of ethyl acetate was added and stirred at room temperature for 3 h;
[0046] 4) Add internal standard 1,3,5-trimethoxybenzene, take a small amount of sample for gas chromatography analysis, and the yield is the gas spectrum yield.
[0047] Table 1 Effect of different catalysts on the yield of N-methyl-N-formylaniline and N,N-dimet...
Embodiment 2
[0051] The influence of embodiment 2 ligand on the yield of formamide and N-methylamines
[0052] A ligand-regulated CO 2 The method for preparing formamide and N-methylamines through selective reduction of amines, the steps are as follows:
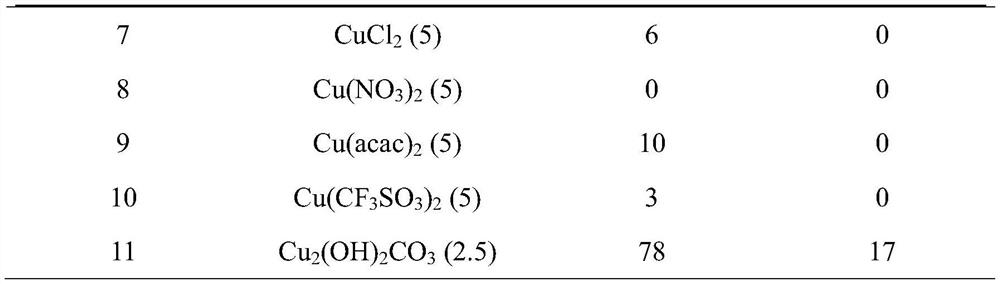
[0053] 1) 2.5mol% catalyst Cu 2 (OH) 2 CO 3 , ligand, acetonitrile 2mL, 54μL (0.5mmol) N-methylaniline, 0.2mL (1.5mmol) phenylsilane were added to a 10mL Schlenk tube, and then a CO 2 the balloon;
[0054] 2) The reaction temperature is controlled at 50°C, and the reaction time is 12h;
[0055] 3) After the reaction was completed and the reaction system was cooled to room temperature, 5 mL of ethyl acetate was added and stirred at room temperature for 3 h;
[0056] 4) Add internal standard 1,3,5-trimethoxybenzene, take a small amount of sample for gas chromatography analysis, and the yield is the gas spectrum yield.
[0057] The influence of table 2 ligand on the yield of N-methyl-N-formylaniline and N,N-dimethylaniline
[0058] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com