A kind of synthetic method of hexaaminobenzene hydrochloride
A technology of hexaaminobenzene hydrochloride and synthesis method, which is applied in the field of synthesis of hexaaminobenzene hydrochloride, can solve the problems of difficult synthesis of HAB, expensive raw materials, easy deterioration of HAB, etc., and achieve reaction safety, green environmental protection, Easy separation and purification, low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
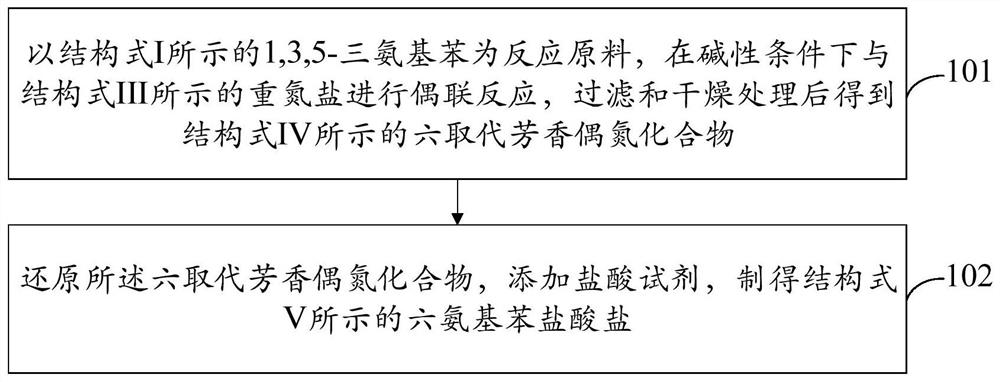
[0069] figure 1 A method flow chart of the preparation method of hexaaminobenzene hydrochloride provided by the embodiment of the present invention is shown. refer to figure 1 , the invention provides a kind of synthetic method of hexaaminobenzene hydrochloride, described method comprises:
[0070] Using the 1,3,5-triaminobenzene shown in structural formula I as the reaction raw material, carry out coupling reaction with the diazonium salt shown in structural formula III under alkaline conditions, and obtain the hexaamine shown in structural formula IV after filtering and drying. Substituted aromatic azo compounds;
[0071]
[0072] The hexa-substituted aromatic azo compound is reduced, and hydrochloric acid reagent is added to obtain the hexaaminobenzene hydrochloride represented by structural formula V.
[0073] Preferably, the method further includes: using nitrite or isoamyl nitrite and an aromatic amine shown in structural formula II to prepare the diazonium salt sh...
Embodiment 1
[0105] Embodiment 1: the easy synthesis of hexaaminobenzene hydrochloride.
[0106] Step 1: Synthesis of aniline diazonium fluoroborate.
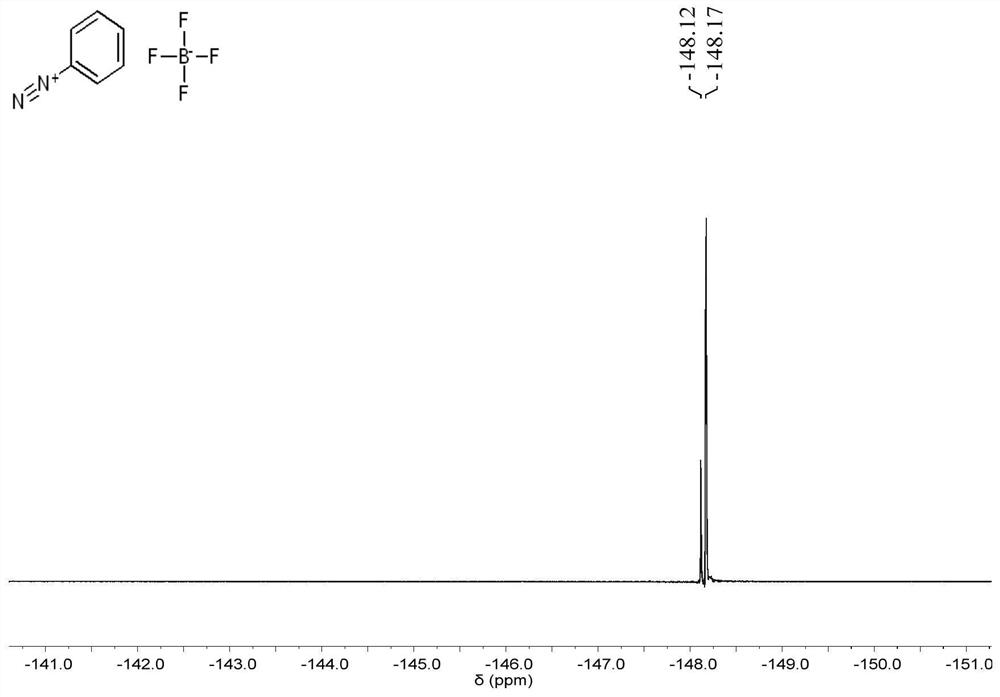
[0107] Sodium nitrite (1.4096g, 21mmol, 1.05eq) was dissolved in 5ml deionized water, and placed in an ice bath for pre-cooling for 10min; after mixing 50wt% tetrafluoroboric acid aqueous solution (6ml) with deionized water (6ml), Place in ice-water mixture for precooling, add aniline (1.8ml, 20mmol, 1eq) dropwise, mix well by magnetic stirring, then add 5ml NaNO dropwise 2 aqueous solution, and reacted for 10 min under magnetic stirring. After the reaction, a white needle-like solid was obtained by filtration, washed with ice water and dried to obtain aniline diazonium fluoroborate (3.3302 g, yield 86.7%).
[0108] refer to figure 2 , image 3 with Figure 4 , shows the proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum and fluorine nuclear magnetic resonance spectrum of the intermediate product an...
Embodiment 2
[0133] Embodiment 2: the easy synthesis of hexaaminobenzene hydrochloride.
[0134] Step 1: Synthesis of 4-methylaniline diazonium fluoborate.
[0135] Sodium nitrite (704mg, 1.01mmol, 1.01eq) was dissolved in 3ml deionized water, and placed in an ice bath for pre-cooling for 10min; after mixing 50wt% tetrafluoroboric acid aqueous solution (3ml) with deionized water (3ml), Pre-cool in an ice-water mixture, add quantitative 4-methylaniline (1.074g, 10mmol, 1eq), stir the mixture uniformly by magnetic stirring, then add 3ml NaNO 2 aqueous solution, and reacted for 10 min under magnetic stirring. After the reaction, a white needle-like solid was obtained by filtration, washed with ice water and dried to obtain 4-methylaniline diazonium fluoroborate (1.8722 g, yield 90.9%).
[0136] refer to Figure 14 with Figure 15 , shows the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum of the intermediate product 4-methylaniline diazonium f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com