Herbicide-resistant ACCase mutant gene and protein and application thereof
A mutant, herbicide technology, applied in the field of plant protein and plant herbicide resistance, can solve the problems that it is difficult to predict in advance the ACCase protein to produce herbicide resistance, and the mechanism of action of the herbicide has not yet been determined.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Determination of rice ACCase gene mutation site
[0062] We used the ACCase sequence (Genbank AJ310767.1) of the plastid of Alopecurus myosuroides (black grass) as a reference sequence, and performed homology alignment with the rice genome, and found that it was identical to the ACCase gene (XM_015783727.1) of the rice genome. ) has the highest homology. According to the amino acid mutation sites mentioned in the patent (CN 102946714 B), the black grass ACCase can be resistant to herbicides, which are 1785 (mutation of alanine to glycine), 1786 (mutation of alanine to proline), 1824 ( Glutamine to proline), 1864 (valine to phenylalanine), 1999 (tryptophan to glycine), 1999 (tryptophan to cysteine), 2039 (glutamine acid to glycine), 2049 (valine to leucine), 2059 (alanine to valine), 2095 (lysine to glutamic acid) as reference, through homologous alignment Methods, the amino acid sites of ACCase gene mutation in rice were identified as 1796 (alanine to glyci...
Embodiment 2
[0063] Example 2: Obtaining the full-length gene of wild-type ACCase of rice Jiahua No. 1
[0064] The leaves of the wild-type plant of Jiahua No. 1 were selected, the genomic DNA was extracted, and the primers were designed according to the ACCase gene of NCBI Nipponbare (NCBI: XM_015783727) for amplification, and KOD DNA polymerase (purchased from Toyobo) was used to amplify the ACCase gene. The reaction system is as follows:
[0065]
[0066] Add sterile water to a total volume of 50 μl
[0067] The PCR amplification reaction procedure adopts a two-step method, annealing and extension are combined together at 68 degrees.
[0068] The procedure was as follows: pre-denaturation: 94°C for 3 min; 35 cycles: denaturation at 94°C for 10 sec; extension at 68°C for 10 min; incubation: 72°C for 10 min.
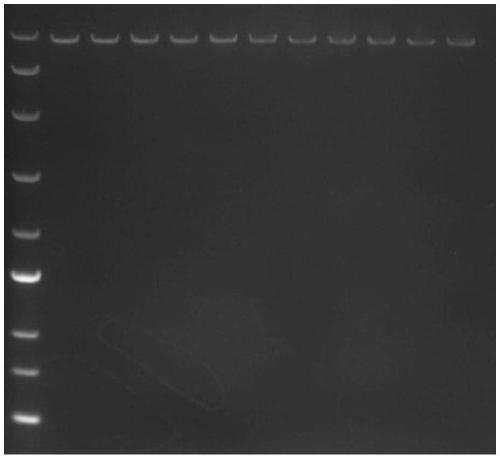
[0069] Take 2 μl of PCR product and test it by 1% agarose gel electrophoresis, after finding fragments of expected size ( figure 2 ), the remaining PCR products were cleaned ...
Embodiment 3
[0070] Example 3: Obtaining the Gene Sequence of Rice ACCase Gene Mutation Site
[0071] The mutation site sequence of rice ACCase gene was amplified by point mutation kit. First, using the primers ACC-CF and ACC-CR, and using Jiahua No. 1 cDNA as a template, the full-length ACCase cDNA was cloned, and ligated into the TK007 vector (Nanjing Qingke Biotechnology Co., Ltd.) to transform E. coli TOP10 strain competent cells. The positive transformants were selected and verified by sequencing, and the correct single clone was obtained, and the plasmid was named TK007-ACCase; secondly, the mutant primers ACC-MutF and ACC-MutR were used, and TK007-ACCase was used as the template for PCR amplification, and the amplification product was restricted. After digestion with endonuclease DpnI, it was transferred into competent cells of Escherichia coli DMT strain, and positive transformants were selected for sequencing verification to obtain a plasmid with ACCase target site mutation.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com