Wood frog antimicrobial peptide gynecological gel
A technology of peptide gynecology and antibacterial peptides, applied in antibacterial drugs, antifungal agents, peptide/protein components, etc., can solve problems such as disrupting the balance of vaginal flora, difficult to cure, and stubborn gynecological diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Rana antimicrobial peptide gynecological gel is made of the following raw materials by weight percentage: 40% Rana antimicrobial peptide concentrate, 5% Cnidium chinensis extract, 5% Sophora flavescens extract, 5% Phellodendron phellodendri extract, and Comfrey extract 3%, dandelion extract 3%, centipede extract 3%, red peony extract 3%, honeysuckle extract 3%, borneol 0.3%, carbomer 1.5%, chitosan 1%, carboxymethyl cellulose Sodium 0.2%, Glycerin 15%, Ethyl Paraben 0.1%, Triethanolamine 1.5%, Sodium Hydroxide 1%, Purified Water 9.4%.
experiment example 1
[0047] Rana antibacterial peptide gynecological gel
[0048] 1. Raw materials:
[0049] Rana Antibacterial Peptide Concentrate 40% Cnidium Fructus Extract 5%
[0050] Sophora flavescens extract 5% Phellodendron phellodendron extract 5%
[0051] Comfrey Extract 3% Dandelion Extract 3%
[0052] Baibu Extract 3% Radix Paeoniae Rubra Extract 3%
[0053] Honeysuckle extract 3% borneol 0.3%
[0054] Carbomer 1.5% Chitosan 1%
[0055] Sodium Carboxymethyl Cellulose 0.2% Glycerin 15%
[0056] Ethylparaben 0.1% Triethanolamine 1.5%
[0057] Sodium hydroxide 1% Purified water 9.4%.
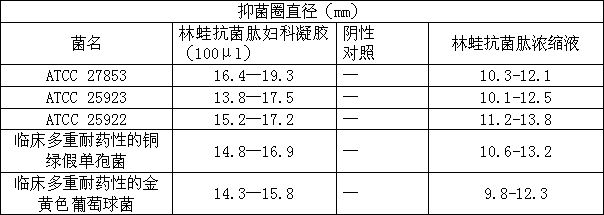
[0058] 2. Zone of inhibition test
[0059] The following test is to use the Rana antimicrobial peptide gynecology gel configured in Example 1.
[0060] (1) Strains
[0061] Pseudomonas aeruginosa ATCC 27853.
[0062] Escherichia coli ATCC 25922.
[0063] Staphylococcus aureus ATCC 25923.
[0064] Pseudomonas aeruginosa with clinical multi-drug resistance (the main drug-resistant names are piper...
experiment example 2
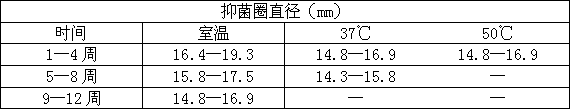
[0073] Stability test: Example 1 formulation.
[0074] Place the present invention at room temperature, 37°C, and 50°C respectively for 3 months, 2 months, and 1 month, and use the above method to do the antibacterial test every week. The bacterial species is Pseudomonas aeruginosa with clinical multidrug resistance. bacteria.
[0075]
[0076] The conclusion is that the patented product of the present invention has a stable antibacterial function through accelerated tests.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com